In this Editor’s choice post, Professor Christer Aakeröy, Associate Editor for CrystEngComm, talks about his favourite articles published in the journal in recent months. Christer has chosen the most interesting articles in the area of molecular recognition crystal engineering.
Deprotonation of resorcinarenes by mono- and diamine bases: Complexation and intermolecular interactions in the solid state
N. Kodiah Beyeh, Arto Valkonen and Kari Rissanen
CrystEngComm, 2013, DOI: 10.1039/C3CE42291G
Structural chemistry investigations of macrocyclic compounds such as calixarenes and resorcinarenes are frequently hampered due to problems with crystal growth, and it is quite rare that a successful structural study is supported and complemented by solution- and gas-phase data. Rissanen and co-workers have produced a systematic and comprehensive exploration of several deprotonated alkyl-resorcinarenes which offers a broad perspective of the structural and chemical behavior of representatives of a ‘classic’ supramolecular system. The single-crystal studies indicate that the deprotonated macrocycles act as effective hosts for the protonated amines in the solid state and the acid-base behavior in solution is monitored by NMR titrations. The deprotonated resorcinarenes (monomer and dimer) were observed using EI mass spectrometry in negative ion mode while the 1:1 and 2:1 complexes with the protonated guests were observed in positive ion mode in the gas phase.
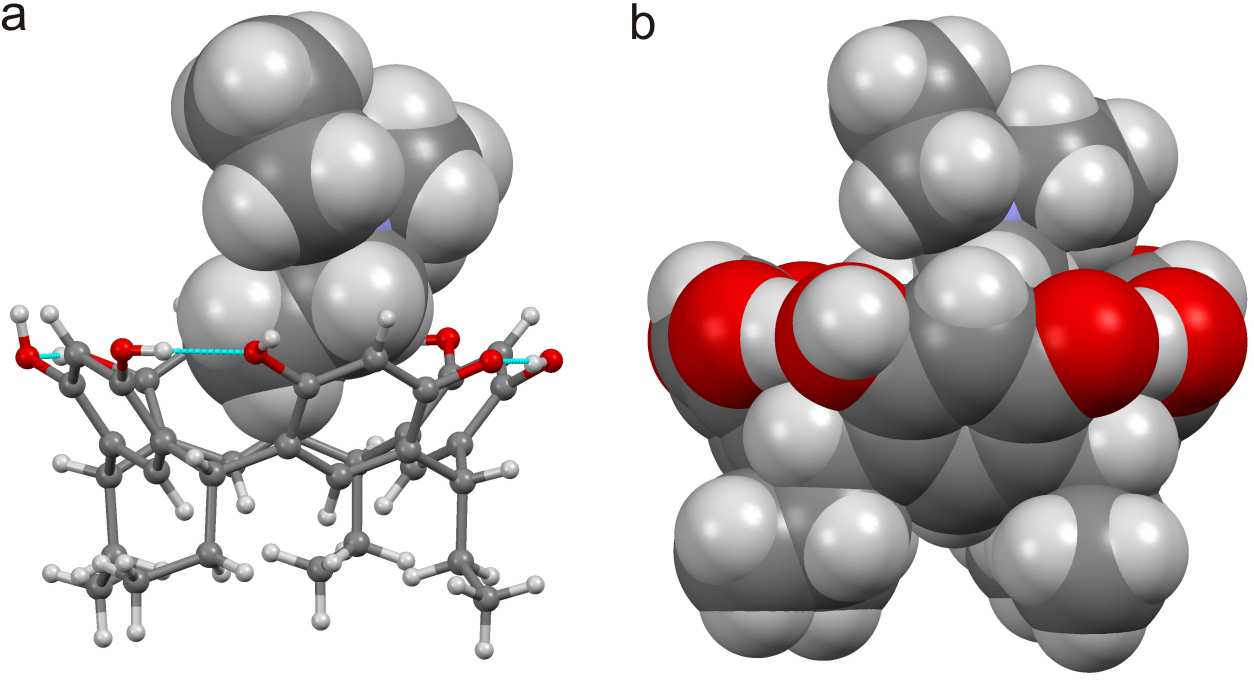
Can self-assembly of copper(II) picolinamide building blocks be controlled?
Marijana Đaković, Diogo Vila-Viçosa, Nuno A. G. Bandeira, Maria José Calhorda, Bojan Kozlevčar, Zvonko Jagličić and Zora Popović
CrystEngComm, 2013, 15, 8074-8087
In this paper, Đaković et al examine the structural role played by picolinamide ligands in the directed assembly of a series of Cu(II) based complexes. The study is supported by an impressive range of experimental and theoretical tools, and offers a comprehensive analysis and evaluation of an important aspect of crystal engineering. The authors have examined the precise role that different factors play in the assembly of each structural motif and the outcome is a much enhanced understanding of the templating effect of the metal ion as well as of the complementarity of non-covalent interactions. Finally, the structural work is supported by careful magnetic measurements the results of which are subsequently properly interpreted and rationalized using DFT-based calculations.
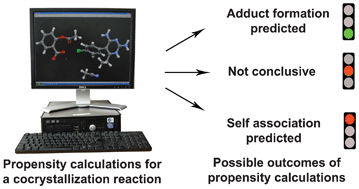
Knowledge-based hydrogen bond prediction and the synthesis of salts and cocrystals of the anti-malarial drug pyrimethamine with various drug and GRAS molecules
Amit Delori, Peter T. A. Galek, Elna Pidcock, Mohit Patni and William Jones
CrystEngComm, 2013,15, 2916-2928
The ability to determine which primary intermolecular interactions are most likely to take place given the presence of certain functional groups is of key importance in crystal engineering. In this study, the authors adopt a knowledge-based approach that takes full advantage of the unique structural information that is provided within the Cambridge Structural Database. Through the use of hydrogen bond propensity calculations (HBPC), which perform a statistical analysis of the occurrence of specific hydrogen bonds among structures of relevant molecules, it is possible to estimate which hydrogen bonds are most likely to take place between different molecules. Different hydrogen bonds are assigned propensity scores which provides an avenue for predicting of co-crystals are likely to form between two different molecules and, if so, the type of intermolecular interactions that can be expected within the heteromeric co-crystal. HBPC calculations were utilized to predict the likelihood of forming co-crystals between the anti-malarial drug pyrimethamine and carbamazepine, theophylline, aspirin, α-ketoglutaric acid, saccharin, coumaric acid, succinimide and L-isoleucine. The HBPC agreed with the experimental observations indicating that this approach may offer effective and versatile tools for finding new solid forms of high-value chemicals such as pharmaceuticals and agrochemicals.
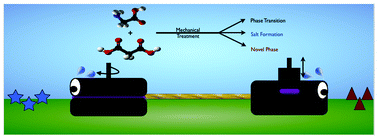
Complexities of mechanochemistry: elucidation of processes occurring in mechanical activators via implementation of a simple organic system
Adam A. L. Michalchuk, Ivan A. Tumanov and Elena V. Boldyreva
CrystEngComm, 2013,15, 6403-6412
Mechanochemistry is a current ‘hot topic’ which is of considerable interest to both covalent and supramolecular synthetic chemists. A wide range of chemical transformations are now known to be possible using essentially ‘green’ reaction conditions. Arguably, most papers that have presented in this field focus on proof-of-principle studies which demonstrate that “functional group ‘A’ can be converted to ‘B’ using the following reaction conditions”, but mechanistic or kinetic studies are still relatively uncommon. In this paper, however, Michalchuk et al, present a very detailed examination of the α-glycine : b-malonic acid system which include an analysis of how two key mechanical ‘forces’ – impact and shear – can influence reaction paths and product distribution. This study clearly demonstrate that what actually happens in a ball mill or through a mortar-pestle treatment is likely to be far more complicated than what is assumed in most studies, notable the role played by the actual mechanoreactor itself. The results are likely to be of critical importance to many mechanochemical reactions especially in the context of scale-up.
Synthon identification in co-crystals and polymorphs with IR spectroscopy. Primary amides as a case study
Arijit Mukherjee, Srinu Tothadi, Shaunak Chakraborty, Somnath Ganguly and Gautam R. Desiraju
CrystEngComm, 2013, 15, 4640-4654
It is probably fair to say that many crystal engineering efforts rely far too heavily on the use of single-crystal X-ray diffraction for product analysis and/or for interpreting the outcome of a particular crystallization or supramolecular synthesis. However, in this paper, Desiraju and co-workers present a careful and informative study that correlates crystallography with vibrational spectroscopy. In order to overcome the well-known challenges when using IR spectroscopy for analyzing polymorphs and co-crystals containing multiple synthons, the authors have employed a four-step approach that relies on the use of ‘spectral markers’ that can be directly associated with bonds in specific synthons. The method is tested on an acid–phenol–pyridine co-crystal system and subsequently utilized for correctly identifying the different synthons in polymorphs of isonicotinamide.

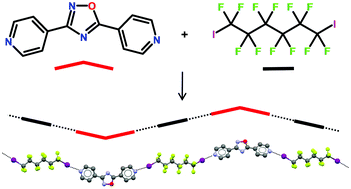
Halogen bond directionality translates tecton geometry into self-assembled architecture geometry
Marco Saccone, Gabriella Cavallo, Pierangelo Metrangolo, Andrea Pace, Ivana Pibiri, Tullio Pilati, Giuseppe Resnati and Giancarlo Terraneo
CrystEngComm, 2013,15, 3102-3105
This paper offers a particularly well-planned and carefully executed strategy for supramolecular synthesis. One some level, this study may seem overly simplistic and the results may appear to be ‘obvious’ but this is, in my opinion, a testament to the progress that has been made in this field. We sometimes forget that non-covalent synthesis has to rely on relatively weak and reversible interactions in an environment where solvent molecules are more than capable of disrupting solute-solute biding and, furthermore, that recrystallization is generally favored over heteromeric co-crystallizations. The fact that we may think that the results presented herein are unsurprising simply means that we are making considerable progress towards finding and developing supramolecular reactions that display a robustness and reliability that we typically associate with named reactions in organic synthesis.