Please take a look at our new batch of HOT articles and enjoy reading in the August sunshine! These are free to access for 4 weeks only!
Our HOT articles have also been compiled into a collection and are available for viewing on our website
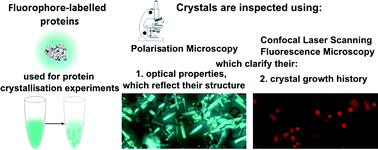
Illuminating protein crystal growth using fluorophore-labelled proteins
Alaa Adawy, Willem J. P. van Enckevort, Elisabeth S. Pierson, Willem J. de Grip and Elias Vlieg
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE01281J
Free to access until 17th October 2014
Towards understanding P-gp resistance: a case study of the antitumour drug cabazitaxel
U. Baisch and L. Vella-Zarb
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE01279H
Free to access until 17th October 2014
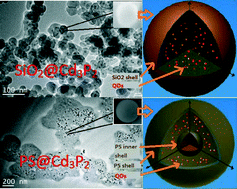
Encapsulated Cd3P2 quantum dots emitting from the visible to the near infrared for bio-labelling applications
Liping Ding, Shulian He, Dechao Chen, Mei Huang, Jinzhang Xu, Stephen G. Hickey, Alexander Eychmüller, Shu-Hong Yu and Shiding Miao
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE01041H
Free to access until 11th September 2014
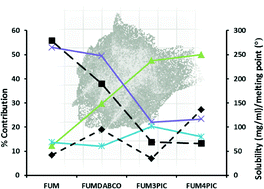
Melting point–solubility–structure correlations in multicomponent crystals containing fumaric or adipic acid
Eustina Batisai, Alban Ayamine, Ornella E. Y. Kilinkissa and Nikoletta B. Báthori
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE01298D
Free to access until 11th September 2014
Cocrystals of telmisartan: characterization, structure elucidation, in vivo and toxicity studies
Renu Chadha, Swati Bhandari, Jamshed Haneef, Sadhika Khullar and Sanjay Mandal
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE00797B
Free to access until 9th September 2014
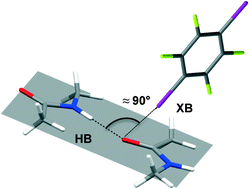
Orthogonal halogen and hydrogen bonds involving a peptide bond model
Vera Vasylyeva, Susanta K. Nayak, Giancarlo Terraneo, Gabriella Cavallo, Pierangelo Metrangolo and Giuseppe Resnati
CrystEngComm, 2014, 16, 8102-8105
DOI: 10.1039/C4CE01514B
Free to access until 9th September 2014














 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.