It gives us great pleasure to assemble a series of issues of CrystEngComm that celebrates 2014 as the International Year of Crystallography (IYCr2014).
Since its inception in 1999, CrystEngComm has been the go-to journal for publishing the highest-quality work in crystal engineering. It was the first online-only journal of the Royal Society of Chemistry and was among the very first to spur and foster an online scientific community.
During the last 15 years, CrystEngComm has witnessed and ushered explosive growth in the field of crystal engineering, as demonstrated by the increase in number of articles published from 9 in 1999 to 1325 in 2013. Indeed, the field of crystal engineering itself now has a highly-successful Gordon Research Conference (GRC) devoted to the subject. Clearly, the launch of CrystEngComm has supported crystal engineering and its numerous sub-areas as the field has developed.
A celebration provides an opportunity to both reflect and look forward. I personally recall travelling as an undergraduate student on a flight to a Chemical Institute of Canada Conference in 1992 and sitting next to a professor who was also travelling to attend the meeting. At that time, I was just beginning to learn how to grow single crystals, as well as collect and solve X-ray data. Our experiments in those days were conducted on a conventional Enraf-Nonius CAD-4 point-detector diffractometer. Data collection times were on the order of days to weeks.
While I had only been a researcher for about one year, in a conversation with the professor I was comfortable enough to remark that, “You really gain a great deal of confidence about chemistry once you have determined a crystal structure”. Clearly, I was already ‘hooked’, at an early stage, by the process of gathering and analysing X-ray data and the insight gained from the X-ray experiment.
Over the past 15 years, CrystEngComm has made many strides scientifically, meaning that we are able to readily draw on an international community to celebrate IYCr2014. To that end, we have assembled a series of issues edited by prominent researchers in crystal engineering that provide a global celebration from regions including:
Each issue contains a series of papers that reflects the wide breath and scope of crystal engineering and modern crystallographic techniques being studied in each region.
Our deepest thanks and gratitude are extended to the Guest Editors (pictures L-R below):
- Michaele Hardie, University of Leeds, UK (CrystEngComm Editorial Board Member)
- Dario Braga, University of Bologna, Italy (first CrystEngComm Scientific Editor)
- Rahul Banerjee, CSIR-National Chemical Laboratory, India (CrystEngComm Associate Editor)
- J. J. Vittal, National University of Singapore, Singapore
- Stuart Batten, Monash University, Australia
- Christer Aakeröy, Kansas State University, USA (CrystEngComm Associate Editor)
- Tomislav Friščić, McGill University, Canada (CrystEngComm Editorial Board Member)
As well as the CrystEngComm staff, for their dedication and hard work to bring such a global effort together.

Guest Editors of IYCr themed issues
All four IYCr14 themed issues have been very successful and I encourage you to take an opportunity to review all of them.
Much change has been realized in crystal engineering and X-ray diffraction during the past two decades. A major change in this regard has been the implementation of charge-coupled device (CCD) detectors, which enable typical data collection times on the order of hours versus days or weeks. The number of structures in the Cambridge Structural Database (CSD) has increased from roughly 200,000 to 700,000 during the time period (one million is coming quickly), which provides the all-important data for crystal engineers to develop improved understandings of structural relationships between solids.
Moreover, with data collection times becoming shorter and shorter, the field of crystal engineering can be expected to deliver even greater insights into the structures and dynamics of crystalline solids. Let us keep each other posted in CrystEngComm.

Leonard R. MacGillivray, Chair, CrystEngComm Editorial Board
Comments Off on Celebrating the IYCr












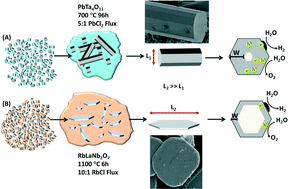
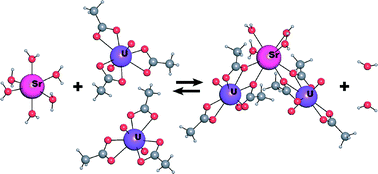
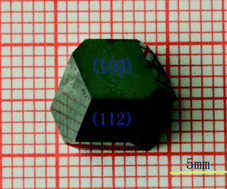

 Rachel Coulter is currently working on a PhD at the University of Liverpool investigating near infrared absorbing materials. Her interests include solvothermal synthesis, optical applications of inorganic compounds and synthesis of nanoparticles. She received an MChem from the University of Edinburgh in 2011, which included an Erasmus year in Lille, France.
Rachel Coulter is currently working on a PhD at the University of Liverpool investigating near infrared absorbing materials. Her interests include solvothermal synthesis, optical applications of inorganic compounds and synthesis of nanoparticles. She received an MChem from the University of Edinburgh in 2011, which included an Erasmus year in Lille, France.