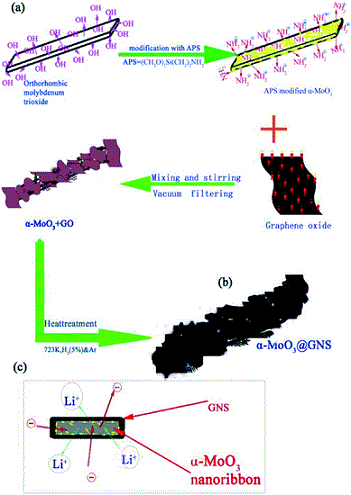
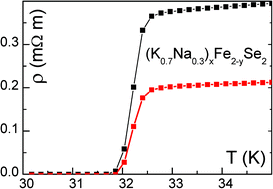
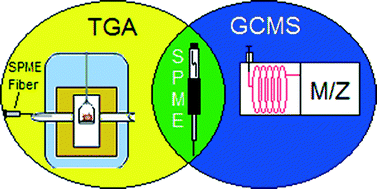
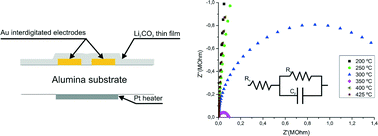
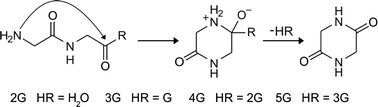
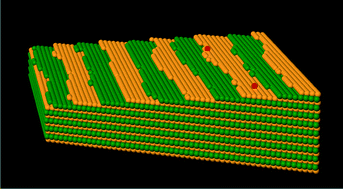
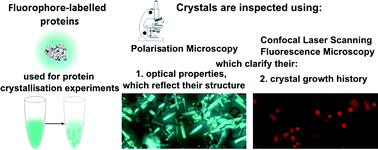
The quality of protein crystals can sometimes make study of their structure by X-ray crystallography challenging. Producing higher quality crystals could be facilitated by a better understanding of the crystal growth process.
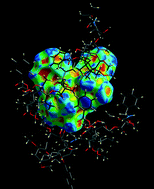
One method of achieving this involves inserting a small fluorescent dye into a protein (to form an F-protein) and adding this labelled protein to the crystallising protein. The distribution, orientation and incorporation efficiency of the F-protein during the growth of the crystals can be studied optically using techniques including polarisation microscopy (see diagram below).
A new paper describes the use of three different F-proteins, each incorporating the dye DY-632-01 NHS ester, to study the crystallisation of the three unlabelled proteins. This enabled visualisation of the crystal growth habits, symmetry and history as well as the distribution of the F-proteins.
In some cases, the F-proteins are preferentially incorporated into the growing crystal and can act as tracers. Alternatively, they may not be incorporated at all and provide information about the biophysical factors which affect crystal growth (such as charge distribution and hydrophobicity).
As different F-proteins behave differently during the crystallisation of a given unlabelled protein, repeating the crystallisation experiment with different F-proteins can provide complementary information.
The distribution of F-proteins is not uniform throughout the crystal and authors conclude that that the diffraction quality of the crystal is position dependent. The incorporation of F-proteins may allow areas of higher diffraction quality to be identified.
For more information, read the full paper:
Illuminating protein crystal growth using fluorophore-labelled proteins
Alaa Adawy, Willem J. P. van Enckevort, Elisabeth S. Pierson, Willem J. de Grip and Elias Vlieg
CrystEngComm, 2014, DOI: 10.1039/C4CE01281J
__________________________________________________________________________________________________
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors












 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.