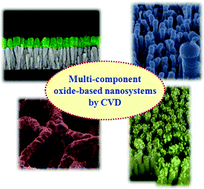
Chemical Vapour Deposition-based growth can help tailor the properties of oxide-based nanomaterials.
Davide Barreca and colleagues survey the current literature on multi-component oxide nanosystems obtained by Chemical Vapour Deposition (CVD) in their HOT Highlight article. Three bi-component categories of nanomaterials are discussed in detail; these are metal/oxide, oxide/oxide and carbon/oxide systems.
The metal/oxide materials include zinc oxide-based systems which are some of the most investigated composites obtained by CVD-based methods. Tin dioxide nanowires covered by iron oxide nanocrystals are an example of the oxide/oxide nanomaterials examined, with CVD techniques being critical for obtaining interconnected magnetite superstructures. A combined plasma enhanced-CVD and electrodeposition approach to create nanofibers coated with manganese oxide is an example of a carbon/oxides systems.
Future challenges include controlling phenomena occurring at interfaces between the materials but the flexibility of CVD techniques can help with overcoming these to further exploit and develop novel oxide nanosystems. Download the Highlight today to find out more; it’s free for 4 weeks.
Multi-component oxide nanosystems by Chemical Vapor Deposition and related routes: challenges and perspectives
Daniela Bekermann, Davide Barreca, Alberto Gasparotto and Chiara Maccato
CrystEngComm, 2012
DOI: 10.1039/C2CE25624J, Highlight
Here are some other articles from the team that you might find interesting…
Controlled synthesis and properties of beta-Fe2O3 nanosystems functionalized with Ag or Pt nanoparticles
Giorgio Carraro, Davide Barreca, Elisabetta Comini, Alberto Gasparotto, Chiara Maccato, Cinzia Sada and Giorgio Sberveglieri
CrystEngComm, 2012
DOI: 10.1039/C2CE25956G, Paper
Strongly oriented Co3O4 thin films on MgO(100) and MgAl2O4(100) substrates by PE-CVD
Davide Barreca, Anjana Devi, Roland A. Fischer, Daniela Bekermann, Alberto Gasparotto, Marco Gavagnin, Chiara Maccato, Eugenio Tondello, Elza Bontempi, Laura E. Depero and Cinzia Sada
CrystEngComm, 2011, 13, 3670-3673
DOI: 10.1039/C1CE05280B, Communication
Malonate complexes of dysprosium: synthesis, characterization and application for LI-MOCVD of dysprosium containing thin films
Andrian P. Milanov, Rüdiger W. Seidel, Davide Barreca, Alberto Gasparotto, Manuela Winter, Jürgen Feydt, Stephan Irsen, Hans-Werner Becker and Anjana Devi
Dalton Trans., 2011, 40, 62-78
DOI: 10.1039/C0DT00455C, Paper
Are you following us on Twitter? @crystengcomm











![GA[11] Applications of halogen bonding](https://blogs.rsc.org/ce/files/2012/09/GA11.gif)

 Nacre
Nacre![GA[6] Ethambutol dibenzoate trimorphs](https://blogs.rsc.org/ce/files/2012/08/GA6.gif)