Posted on behalf of Jamie Humphrey, Editor
 Crystallography is a core science, underpinning many areas of research, and central to crystal engineering. 2014 marks the centenary of the birth of X-ray crystallography and has been proclaimed the International Year of Crystallography. We are delighted to celebrate this important science in CrystEngComm in a number of ways in 2014.
Crystallography is a core science, underpinning many areas of research, and central to crystal engineering. 2014 marks the centenary of the birth of X-ray crystallography and has been proclaimed the International Year of Crystallography. We are delighted to celebrate this important science in CrystEngComm in a number of ways in 2014.
Our celebrations will take the form of the publication of four themed issues dedicated to the best crystal engineering research from four key geographical regions. Keep an eye out for these issues, which will appear throughout the year, covering India (Guest Editor: Professor Rahul Banerjee, CSIR-National Chemical Laboratory, Pune, India), Europe (Guest Editors: Professor Dario Braga, University of Bologna, Italy and Professor Michaele Hardie, University of Leeds, UK), the Americas (Guest Editors: Professor Christer Aakeröy, Kansas State University, USA and Professor Tomislav Friščić, McGill University, Canada) and Asia-Pacific (Guest Editors: Professor Stuart Batten, Monash University, Australia and Professor Jagadese Vittal, National University of Singapore, Singapore).
We also have a number of excellent topic-themed issues planned for the year, illustrating the breadth of crystal engineering research: Functional co-crystals (Guest Editor: Professor Colin Pulham, University of Edinburgh, UK), Structural macrocyclic supramolecular chemistry (Guest Editors: Professor Len Barbour, University of Stellenbosch, South Africa, Professor Len MacGillivray, University of Iowa, USA and Professor Kari Rissanen, University of Jyväskylä, Finland), Nanoparticle assemblies (Guest Editors: Professor Marie Paule Pileni, UPMC, Paris, France, Professor P. Davide Cozzoli, Università del Salento & National Nanotechnology Laboratory, Italy and Professor Nicola Pinna, Humboldt-Universität zu Berlin, Germany), and Nanocrystal growth via oriented attachment (Guest Editors: Dr R. Lee Penn, University of Minnesota, USA, Dr Hengzhong Zhang, University of California, Berkeley, USA, Dr Zhang Lin, State Key Laboratory of Structural Chemistry, China and Dr Helmut Cölfen, University Konstanz, Germany). These follow the themed issues published in 2013, details of which are in Table 1.
Table 1 2013 themed issues, representing the breadth of crystal engineering
| Themed issue |
Guest editor(s) |
| Covalent organic frameworks |
Andy Cooper, University of Liverpool, UK |
| Crystallisation: from fundamentals to application |
Alastair Florence, University of Strathclyde, UK |
| Halogen bonding: from self-assembly to materials and biomolecules |
William T Pennington, Clemson University, USA |
| Giuseppe Resnati, Politecnico di Milano, Italy |
| Mark S Taylor, University of Toronto, Canada |
| MOFs |
George Shimizu, University of Calgary, Canada |
| Rahul Banerjee, CSIR-National Chemical Laboratory, India |
| Miao Du Tianjin, Normal University, China |
| NMR crystallography |
John Ripmeester, National Research Council, Canada |
| Roderick E. Wasylishen, University of Alberta, Canada |
Continuing the International Year of Crystallography celebrations, the Royal Society of Chemistry, in partnership with Calcutta University, Jadavpur University and IISER-Kolkata, will organise a symposium on structural chemistry in November in India. We will also participate in a global experiment for school children involving crystals. Keep an eye out for more information about the symposium and the global experiment as they develop.
In 2013 a number of people joined our Editorial Board, bringing with them expertise and experience of their particular research fields. Joining Editorial Board Chair, Len MacGillivray (University of Iowa, USA), Associate Editors Rahul Banerjee (CSIR-National Chemical Laboratory, India) and Christer Aakeröy (Kansas State University, USA) and Board member Nicola Pinna (Humboldt-Universität zu Berlin, Germany) are Associate Editors Hongjie Zhang (Changchun Institute of Applied Chemistry, China) and Georg Garnweitner (TU Braunschweig, Germany) and Board members Graeme Day (University of Southampton, UK), Tomislav Friščić (McGill University, Canada), Michaele Hardie (University of Leeds, UK), Helmut Cölfen (Universitat Konstanz, Germany) and Pierangelo Metrangolo (Politecnico di Milano, Italy). We very much look forward to our collaborations with them all.
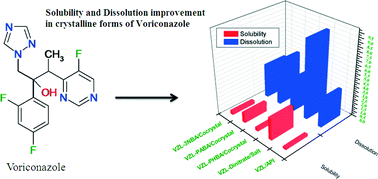
The way we connect with our friends, peers and colleagues is changing, and social media is an important tool for this. CrystEngComm is no different. We have a number of outlets for CrystEngComm via social media, expanding the ways in which people can get involved with the journal. The journal has a Facebook page and Twitter account (why not join our 1000 followers?) and our LinkedIn group is a good way to learn about relevant conferences and other news. Our latest offering is our ‘Crystal Clear’ Pinterest page, illustrating some of the stunning crystal images that are included in the journal.
Fiona McKenzie (Deputy Editor) and I will be attending two conferences in 2014: the Crystal Engineering Gordon Research Conference and the IUCr meeting. An important related meeting is the Faraday Discussion meeting 170 with a focus on Mechanochemistry: From Functional Solids to Single Molecules. The meeting will be in Montreal, Canada in May, and more details are available via www.rsc.org/ConferencesAndEvents/RSCConferences/FD/FD170/index.asp
We were very pleased to support a number of international conferences in 2013 through sponsorship. We also provide support via poster prizes – in 2013, we awarded 10 poster prizes, celebrating the achievements of members of our community in the early years of their academic careers. If you are organising a conference in 2014 and you would like us to sponsor a poster prize, please let us know.
Let us connect you and your work into the world’s leading chemistry community in 2014, the International Year of Crystallography: publish with CrystEngComm!
Have a fantastic 2014!
Download the pdf here



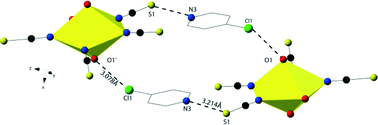










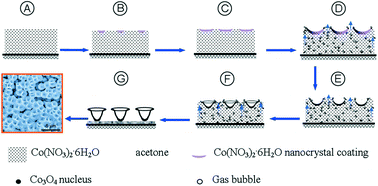

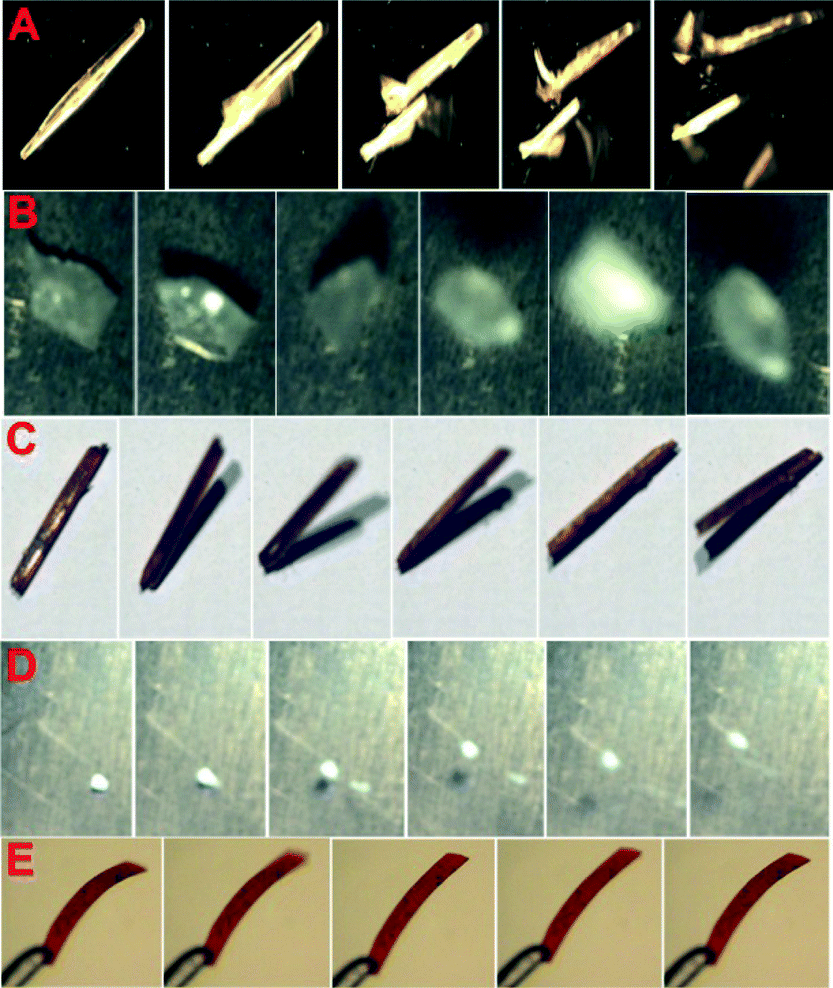 A
A 
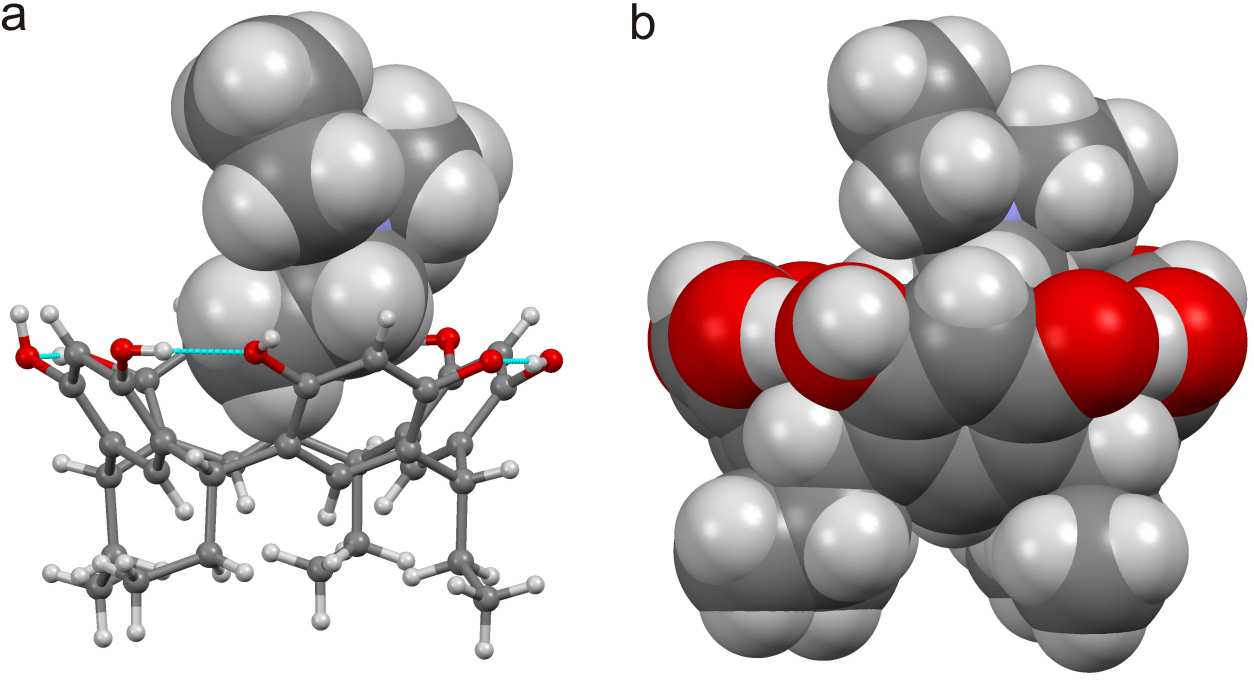
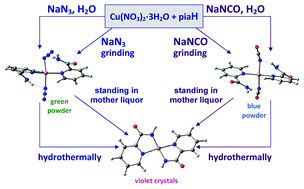
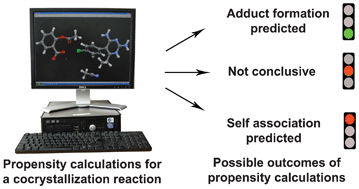
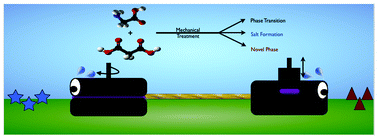

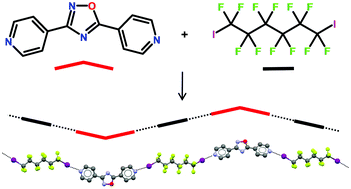 .
. 

 Crystallography is a core science, underpinning many areas of research, and central to crystal engineering. 2014 marks the centenary of the birth of X-ray crystallography and has been proclaimed the International Year of Crystallography. We are delighted to celebrate this important science in CrystEngComm in a number of ways in 2014.
Crystallography is a core science, underpinning many areas of research, and central to crystal engineering. 2014 marks the centenary of the birth of X-ray crystallography and has been proclaimed the International Year of Crystallography. We are delighted to celebrate this important science in CrystEngComm in a number of ways in 2014.