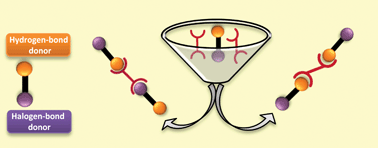
In this HOT article by Aakeröy et al, relevant hydrogen-bond and halogen-bond acceptors and donors were selected to study the competition between these two molecular interactions in supramolecular assembly. Twelve co-crystals were synthesized based on two different azobipyridines as acceptors and six donor molecules possessing both hydrogen-bond and halogen-bond moieties. The key conclusions which could be drawn from the study were: (i) the hydrogen-bond is the principal driving force in the formation of 3,3′-azabipyridine co-crystals whereas hydrogen and halogen bonds do indeed compete in the formation of the 4,4′-azabipyridine co-crystals; (ii) the iodine donor appears to be a better halogen-bond donor than bromine; and (iii) the three different hydrogen-bond donors studied (–COOH, –OH and –CN(R)OH) behave similarly.
Read more for FREE at:
Competing hydrogen-bond and halogen-bond donors in crystal engineering
Christer B. Aakeröy , Sheelu Panikkattu , Prashant D. Chopade and John Desper
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C2CE26747K
This article will be published in a forthcoming themed issue on Halogen bonding, other highlights include:
Iodo-imidazolium salts: halogen bonding in crystals and anion-templated pseudorotaxanes
Antonio Caballero, Sam Bennett, Christopher J. Serpell and Paul D. Beer
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C2CE26020D
Halogen bonding at work: recent applications in synthetic chemistry and materials science
Franck Meyer and Philippe Dubois
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C2CE26150B