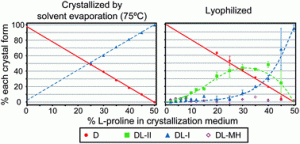
The particular form a crystalline drug takes is highly relevant in the pharma industry as different crystal forms often have different properties such as dissolution rates or stabilities. Though crystallisation processes are commonplace in the production of enantiopure drugs, the way in which one enantiomer can affect the crystallisation of another has not be thoroughly studied.
In their recent CrystEngComm Hot Article, Munson and Berendt carry out such a study investigating proline enantiomers in a range of enantiomeric ratios. They determine that both the enantiomeric ratio and crystallization method influenced the polymorphism of the racemic cocrystal. Read how their work will have implications for current polymorphic screening methods used in the pharma industry in their article:
Effect of enantiomeric ratio and preparation method on proline crystal form
Robert T. Berendt and Eric J. Munson
CrystEngComm, 2012, DOI: 10.1039/C2CE06445F