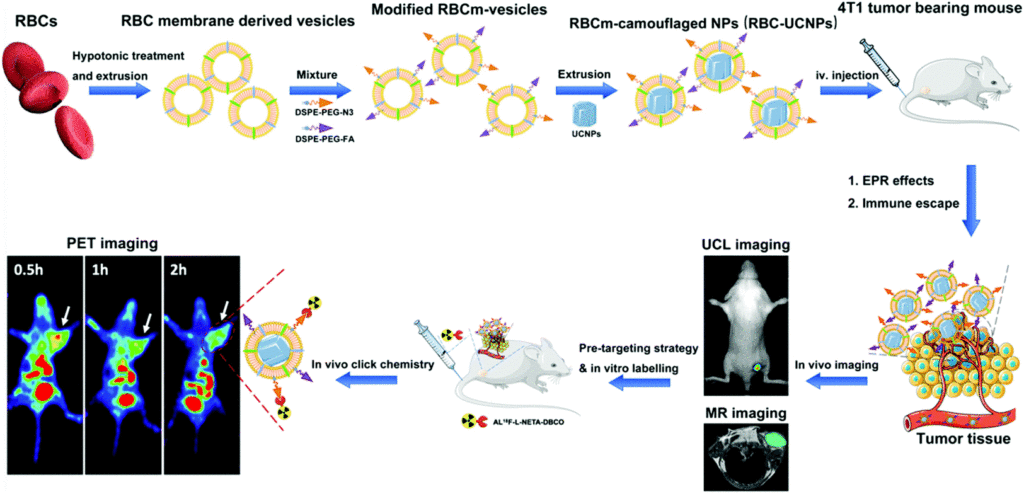
Managing cancer requires visualization of tumors using a plethora of imaging modalities such as positron-emission tomography (PET), magnetic resonance imaging (MRI), computed tomography (CT), photoacoustic tomography and optical imaging. Upconversion nanoparticles (UCNPs), a new generation of optical nanomaterials which convert near-infrared (NIR) radiation to visible light by a process called “upconversion luminescence” (UCL), are garnering a lot of attention in cancer diagnostics due to their ability to selectively label cancer cells.
Researchers from Suzhou, China have recently coated tumor targeting UCNPs with red blood cell (RBC) membranes to render them stealthy, effectively preventing them from immune attack and clearance by the host system. Subsequently, they assessed the utility of these RBC-UCNPs for targeted multimodality imaging of 4T1 breast cancer, a triple-negative breast cancer.
First, they isolated cell membranes from the RBCs, reconstructing them into vesicles which were used to encapsulate UCNPs via extrusion. Folic acid (FA) molecules were inserted into the surface of these RBC-UCNPs to assess the tumor-targeting ability of nanoparticles. Upconversion fluorescence imaging revealed that RBC-FA-UCNPs intravenously injected into mice bearing 4T1 subcutaneously transplanted tumors exhibited quick accumulation, long-term retention and reduced uptake by the immune system.
Next, they investigated the feasibility of using these biomimetic nanoparticles in MRI and PET imaging for the detection of tumors in vivo. They found that the MR signal was significantly enhanced by the FA-RBC-UCNPs, indicating the increased circulation time of particles at the tumor site. Furthermore, a combination of pre-targeting strategy and in vivo click chemistry was utilized to mediate PET imaging, which indicated that the biomimetic nanoparticles displayed a higher tumor uptake of the tracer compared with controls, on application of a short half-life radionuclide.
Finally, they conducted in vivo toxicity studies in mice over a span of 30 days, to assess cytotoxicity of the nanoparticles. Blood chemistry, hematology, and histological analyses indicated non-significant induction of toxicity and organ damage, in turn demonstrating the biocompatibility of the biomimetic nanoparticles and their suitability for clinical utilization.
Taken together, the results indicate the potential of this platform for further applications in realizing early diagnosis, bioimaging and treatment of tumors, especially for deep-seated lesions.
To find out more please read:
Mengting Li, Hanyi Fang, Qingyao Liu, Yongkang Gai, Lujie Yuan, Sheng Wang, Huiling Li, Yi Hou, Mingyuan Gao and Xiaoli Lan
Biomater. Sci., 2020,8, 1802-1814, DOI: 10.1039/d0bm00029a