Obtaining real-time visual information and feedback is vital for surgeons while removing tumors located in the brain, where a lack of precision can lead to catastrophic surgical complications and reduced life expectancy. During surgery, the human eye can identify only anatomical structures and is unable to detect features at the molecular level, which makes it challenging for surgeons to differentiate tumor tissue from surrounding normal brain tissue. Fluorescence-guided surgery attempts to overcome this limitation and relies on the administration of a fluorescent dye which accumulates within the tumor and produces light which in turn is captured and visualized using a camera.
Researchers from Guangzhou, China have recently engineered microglial cells into optical imaging agent vehicles, achieving more accurate brain tumor imaging for fluorescence-guided surgery compared with the commercially used tracer 5-aminolevulinic acid (5-ALA).
First, they activated BV2 microglial cells with citric-acid coated iron oxide nanoparticles (CIONPs) and loaded them with near-infrared fluorescent dye DiD (DiDBV2-Fe). Priming the cells with iron oxide nanoparticles downregulated M2 markers associated with the immunoresponse, and upregulated expression levels of genes that promote transportation of cells across the blood–brain barrier (BBB), thus achieving a two-fold favorable effect.
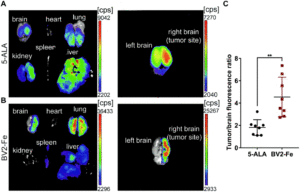
Next, they assessed the administration of DiDBV2-Fe in glioblastoma-bearing mice models via two routes:. intravenous and intracarotid artery injection. The latter route resulted in more efficient accumulation of activated cells in the brain tumor, 2.2 times higher than that of 5-ALA, 8 hours after application. Maximum fluorescence intensity images of brain tissues acquired at various timepoints from 2 to 24 hours using near-infrared imaging revealed clear tumor border demarcation. Confocal microscopy of harvested brain tumor sections showed noticeable co-localization of DiDBV2-Fe with the Ki67 positive tumor cells along with a significantly higher tumor-to-brain fluorescence ratio compared with 5-ALA (4.54 vs. 1.81).
Finally, they evaluated the in vivo preliminary safety of DiDBV2-Fe in comparison to 5-ALA. Administering DiDBV2-Fe did not induce acute liver injury, phototoxic or hypersensitivity reactions until a certain threshold was reached. In addition, the engineered microglia did not induce gene expression changes of the detected immunoregulatory proteins, unlike 5-ALA which induced both phototoxic and photoallergic reactions.
Taken together, the results indicate that these engineered microglial cells can serve as biological homing vehicles – in seeking out tumors and delivering optical imaging agents, which in turn can help surgeons navigate and identify tumor tissue via fluorescence during surgery.
To find out more please read:
Ling Guo, Xiaochen Zhang, Runxiu Wei, Gaojie Li, Bingzhi Sun, Hongbo Zhang, Dan Liu, Cuifeng Wang and Min Feng
Biomater. Sci., 2020, 8, 1117-1126.