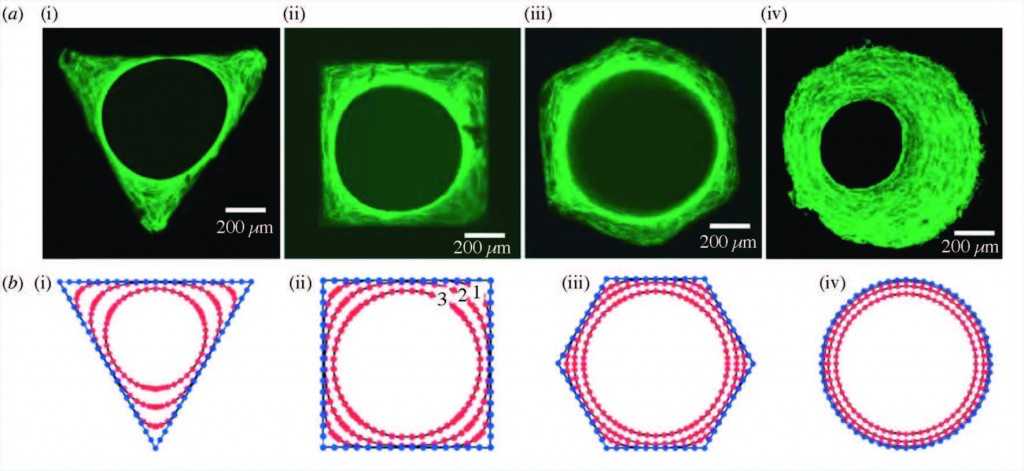
Staining of actin stress fibers to visualize the tissue formed in vitro and to study the effects of curvature (a). The predicted tissue regeneration based on a linear curvature-dependent theoretical model is depicted in subfigure (b). Theoretical predictions match these in vitro experimental observations.
In this paper, Professor Amir Zadpoor reviews the role of biomaterial scaffold geometries on regenerating bone tissue. Scaffold curvature, pore size, and pore shape are all shown to be important in stimulating more bone growth.
In the case of large bone injuries, the role of scaffolds in regenerating bones becomes increasingly critical. The ideal bone scaffolds need to be biocompatible, mechanically strong, and contain pores that allow the transport of nutrients and new cell growth.
The geometry of the scaffold plays a very important role in regenerating bone tissue, and is explored in this paper. Broadly speaking, the curvature of the scaffold, the shape, and the size of the pores are all key components that influence how well the bone is structured and grows.
Strikingly, it has been found that curvature of the surface proportionally impacts the rate of tissue regeneration. Curvature of the surface can be much smaller than, on the scale of, or much larger than cells that grow on the surface. Smaller curvatures can impact individual cell focal adhesions, through which cells can both sense the underlying surface and apply force to it. Larger curvatures can impact cell stress fibers as well as the overall forces on the growing bone tissue.
Pore size can also dramatically impact bone regeneration. It is already known that a limited amount of inflammation is actually good for bone growth. Pore size can affect inflammation – for example, larger pores and wider scaffold geometry angles can increase local inflammation. Pore size can also dictate the amount of oxygen and nutrients reaching the cells, and whether cartilage or bone is formed first.
Pore shape is also extremely important – for example, it has been shown that when cells are grown in scaffolds shaped like parallelograms, alkaline phosphatase activity is increased. Since alkaline phosphatase is a byproduct of bone growth, this data suggests a role for the shape of scaffold pores in bone generation.
However, there are significant caveats to be considered when studying bone regeneration in the lab. Since bone grows in stages, a short in vitro study may not capture all nuances of bone growth that occur in vivo. It is important to study in vitro and in vivo bone generation in parallel to reconcile any contradictory data. Further, it must be noted that it is often difficult to isolate the independent impact of one specific property of a scaffold without affecting another property. For example, changing pore size can also change the overall mechanical properties of the scaffold.
Despite these limitation, it is clear that the geometrical properties of the scaffold can significantly impact bone growth. Computational studies and modeling are also now being used to optimize many scaffold properties, and have the potential to drive further research to elucidate the role of scaffold geometry in clinically valuable bone growth.
Check out the full article:
Bone tissue regeneration: the role of scaffold geometry by Amir A. Zadpoor
Debanti Sengupta completed her PhD in Chemistry in 2012 from Stanford University. She was previously a Siebel postdoctoral scholar at the University of California, Berkeley, and is currently a postdoctoral scholar in Radiation Oncology at Stanford University. Follow her on Twitter @yoginiscientist.
Follow the latest journal news on Twitter @BioMaterSci or go to our Facebook page.











