Ex vivo engineering of 3D organs for transplantation purposes has made tremendous strides in recent years, yet complex tissues remain challenging due to their need for vascularization. A new study takes great steps towards solving this issue using a new, simple bioprinting process to create branched vascular networks capable of effective media exchange. The introduced methodology provides a pathway towards successful incorporation of a branched vasculature into tissue-engineered organs.
The concept of tissue engineering – combining cells with biomaterials to create living, functional tissues – to provide a solution to the lack of suitable organs for transplantation has been tremendously popular for several decades now, and for good reason. It harnesses the potential to not only tailor organ characteristics to individual patients and reduce transplant rejection risks, but also to virtually erase waiting time for patients in need. The combined efforts of numerous researchers have already led to great success in engineering simple tissues such as skin, yet the evolution towards engineering more complex tissues has proven to be highly challenging. A major roadblock has been, and continues to be, the need for nutrient delivery and media exchange for living tissues to survive, let alone thrive. The incorporation of a vascular network is required for this, yet current methods for creating these networks can either not generate efficient, perfusable systems with the necessary mechanical properties, or are too complex to effectively utilize in thick tissues.
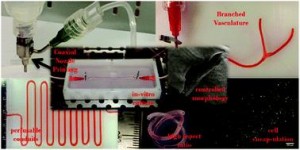
To address the current challenges, Ibrahim Ozbolat’s research group at the University of Iowa designed a novel system for bioprinting vascular conduits. Bioprinting allows for precise 3D fabrication of cell constructs, usually using a sacrificial support biomaterial. In his study, Dr. Ozbolat’s system consists of a coaxial nozzle in which cell-loaded alginate is dispensed through the sheath, and a crosslinking calcium chloride solution through the core, to allow for instantaneous formation of hollow fiber conduits without the need for post-fabrication procedures. The nozzle is under precise robotic control, allowing fabrication of conduits of desired dimension and geometry.
In the current work, the applicability of the bioprinting unit is demonstrated using human umbilical vein smooth muscle cells (HUVSMCs) embedded in alginate as the vessel wall material. By varying alginate and calcium chloride concentrations, conduit properties could be controlled. Relevant properties reported here include vascular lumen and wall dimensions, burst pressures and wall permeability to allow nutrient diffusion. Especially the latter is of paramount importance for long-term functioning of complex engineered tissues. Importantly, this report also shows that despite significant loss of viability during the bioprinting process, alginate-encapsulated cell recovered completely and proliferated well, laying down extracellular matrix throughout the vessel wall as evidenced by histology. Perhaps most intriguing is the demonstrated formation of branched vascular conduits using Dr. Ozbolat’s system.
The straight-forward bioprinting system reported here, allowing for tight control of vascular conduit dimensions, mechanical and perfusion properties, represents a highly promising platform for incorporating effective media exchange and nutrient transport in 3D engineered tissues. Especially the unique lack of post-fabrication requirements and capability for printing branched vessels increase the applicability of this particular design.
In vitro study of directly bioprinted perfusable vasculature conduits
Yahui Zhang, Yin Yu, Adil Akkouch, Amer Dababneh, Farzaneh Dolati and Ibrahim T. Ozbolat
Biomater. Sci., 2015, Advance Article DOI: 10.1039/C4BM00234B
 Robert van Lith is currently a Post-Doc in the Biomedical Engineering department at Northwestern University, developing intrinsically antioxidant biomaterials. He recently received his Ph.D. from Northwestern University for his work on citrate-based antioxidant polyesters, receiving an American Heart Association Fellowship and Society for Biomaterials award for his work. He was trained in the Netherlands, holding an M.S. degree in Biomedical Engineering from Eindhoven University of Technology. Read more about Robert’s research publications here.
Robert van Lith is currently a Post-Doc in the Biomedical Engineering department at Northwestern University, developing intrinsically antioxidant biomaterials. He recently received his Ph.D. from Northwestern University for his work on citrate-based antioxidant polyesters, receiving an American Heart Association Fellowship and Society for Biomaterials award for his work. He was trained in the Netherlands, holding an M.S. degree in Biomedical Engineering from Eindhoven University of Technology. Read more about Robert’s research publications here.












 We hope you enjoy reading our latest
We hope you enjoy reading our latest