The cover story for the July 2014 issue of Biomaterials Science highlights the use of nanovehicle particles for the targeted delivery of chemotherapy drugs to tumor cells in bone.
Bone metastasis, or the spreading of tumor cells from the initial tumor site to bone tissue, marks the stage of cancer progression where the diseased is deemed incurable. Currently, the main method to treat bone metastasis is to utilize aggressive chemotherapy drugs and destroy metastatic tumor cells. Unfortunately, untargeted chemotherapy treatments result in death of both cancerous and healthy tissue. Treatments could be significantly improved with the technology to specifically target the destruction of tumor cells without affecting the surrounding bone tissue.
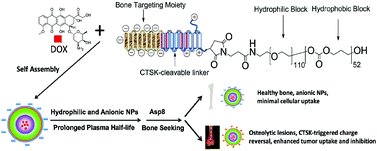
Researchers at the University of Utah, University of Iowa, and Wuhan University in China are making this desired technology a reality. Wang et al. have developed a nanovehicle capable of targeting tumor cells within bone tissue. Nanovehicles (NVs) are essentially nano-sized cargo containers capable of delivering drugs to a desired destination. The cleverly designed NVs are designed to target diseased bone tissue to deliver chemotherapy drugs at a specific site.
The NVs developed in this study have three design features:
(1) The NVs core is made of hydrophilic and hydrophobic block co-polymers able to form a shell-like structure known as a micelle. The micelle serves as the cargo vessel that contains the chemotherapy drug doxorubicin.
(2) The outermost segments of the NVs are negatively charged bone-targeting peptides. The peptide is responsible for the accumulation of NVs in skeletal tissue, and the negative charge prevents cellular uptake of the NVs into healthy tissue.
(3) For uptake into cancer cells, the particle must be positively charged. Therefore, a positively charged peptide linker was added in between the micelle core and the negatively charged bone-targeting region. The peptide linker is cleavable by cathepsin K enzyme (CTSK), which is overexpressed in bone metastatic lesions. When the NV enters a metastatic lesion, the CTSK cleaves off the negatively charged peptides and exposes the positively charged peptides. This charge reversal allows for tumor cells present in the metastatic lesion to uptake the NV.
After extensively characterizing the nanoparticles with NMR and zeta potential measurements, the NVs were used to observe decreased tumor cell viability under CTSK-rich conditions in vitro. More strikingly, in vivo tests of the NVs in a mouse model of bone metastasis showed significant increases in mouse survival and decreases in overall tumor burden.
Collectively, these ingeniously designed nanovehicles show great promise in providing an effective targeted therapy for bone metastasis.
Peptide decoration of nanovehicles to achieve active targeting and pathology-responsive cellular uptake for bone metastasis chemotherapy
X. Wang, Y. Yang, H. Jia, W. Jia, S. Miller, B. Bowman, J. Feng, and F. Zhan
Biomater. Sci., 2014, Advance Article DOI: 10.1039/C4BM00020J
Brian Aguado is currently a Ph.D. Candidate and NSF Fellow in the Biomedical Engineering department at Northwestern University. He holds a B.S. degree in Biomechanical Engineering from Stanford University and a M.S. degree in Biomedical Engineering from Northwestern University. Read more about Brian’s research publications here.
Follow the latest journal news on Twitter @BioMaterSci or go to our Facebook page.