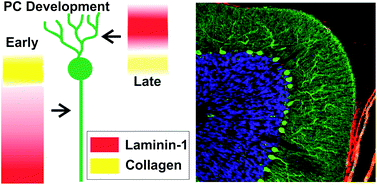
The cerebellum is a region in the brain responsible for regulating motor control and cognitive functions such as attention and language. During development of the cerebellum (and of other tissues), cells interact with the surrounding microenvironment known as the extracellular matrix (ECM). These neuron-ECM interactions regulate neuronal differentiation, growth, formation of synapses, and neurite outgrowth. Specifically, ECM components including collagen and laminin-1 (lam-1) are known to regulate the alignment, migration, and neurite outgrowth of Purkinje cells (PCs). However, the dual signaling roles of collagen and laminin-1 during cerebellar tissue development have not been fully explored.
Dr. Shantanu Sur, a postdoctoral fellow in Prof. Samuel Stupp’s laboratory at Northwestern University, has collaborated with Dr. Thomas Launey at the RIKEN Brain Science Institute in Japan to explore cerebellar tissue development using biomaterial hydrogels. Sur has developed an artificial matrix consisting of collagen and synthetic peptide amphiphile (PA) molecules presenting IKVAV, the peptide on laminin-1 responsible for cell adhesion and neurite outgrowth. Sur et al. evaluated the spatiotemporal expression of lam-1 and collagen in rat cerebellums during PC development (embryonic to post-natal) using histology and immunostaining. Given the changes in the ratio of lam-1 to collagen during PC formation and growth, these results suggest the dynamic involvement of these ECM proteins in forming the neural architecture of the cerebellum.
Using a biomimetic approach to mimic the critical lam-1 to collagen ratio, Sur proceeded to model the dynamic nature of the ECM during PC development with a synthetic collagen-PA hydrogel. Collagen (types I-V) and IKVAV-PA molecules were mixed together in solution at varying concentrations and gelled using ammonia vapor. This simple method allowed Sur to evaluate the density of PC growth, axon guidance, and dendrite morphology in gels using a wide array of collagen and IKVAV-PA concentrations. Strikingly, effects on PC phenotype were observed as a function of the collagen:IKVAV-PA ratio and not the absolute concentrations of each ECM component within the matrix.
Sur comments that the hybrid matrix provides an easily tunable environment to enable the in vitro testing of the role of ECM signals on neuronal maturation. “Our study shows that the optimal ECM-derived cues for neurons change at specific stages of development,” Sur says. “This observation will drive us to work on the design of a dynamic matrix where the extracellular signals delivered to the neuron can be tuned spatiotemporally.” Additionally, the collagen/IKVAV-PA gels may be used to identify cell-ECM interactions during the development of other tissue types, given the simplicity of the technique.
Collectively, this study demonstrates the exciting use of engineered matrices to evaluate spatiotemporal cell-ECM interactions, with the hopes to further elucidate mechanisms of tissue development.
Synergistic regulation of cerebellar Purkinje neuron development by laminin epitopes and collagen on an artificial hybrid matrix construct
Shantanu Sur, Mustafa O. Guler, Matthew J. Webber, Eugene T. Pashuck, Masao Ito, Samuel I. Stupp, and Thomas Launey
Biomater. Sci., 2014, Advance Article, DOI: 10.1039/C3BM60228A
Brian Aguado is currently a Ph.D. Candidate and NSF Fellow in the Biomedical Engineering department at Northwestern University. He holds a B.S. degree in Biomechanical Engineering from Stanford University and a M.S. degree in Biomedical Engineering from Northwestern University. Read more about Brian’s research publications here.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents