Bioactive ceramics are commonly used for the repair and replacement of damaged bone tissues. However, there is still a limited understanding of how the inherent material properties of these ceramics can be used to predict their osteoinductive properties. The development of models which relate these quantities could lead to the more efficient and intelligent design of materials that promote bone formation.
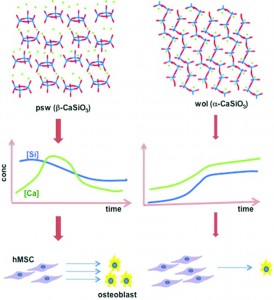
In a study that pushes this goal one step closer to reality, researchers from the University of Wisconsin, Madison, the University of Akron and the University of Michigan investigate the role which crystal structure plays in controlling the attachment and osteogenic differentiation of human mesenchymal stem cells (hMSCs). The group studied hMSC viability and differentiation on two CaSiO3 polymorphs: pseudowollastonite (psw) and wollastonite (wol). As polymorphs, these materials have identical chemical compositions and stoichiometries, and can be fabricated to have nearly equivalent surface roughness. The only significant difference between these two materials is their crystal structure. While the wol polymorph has a stable, open silicate chain structure, the psw polymorph has a highly strained silicate ring structure.
Interestingly, significant differences in hMSC growth and differentiation were observed, with the psw polymorph exhibiting superior osteoinductive properties. Although the hMSCs cultured on psw initially exhibited poor viability, they were able to recover and, after 20 days, exhibited a statistically higher cell count than the hMSCs cultured on wol. Fine-grained calcium phosphate precipitate developed on the surfaces of both polymorphs but only the psw polymorph exhibited polycrystalline calcite aggregates as well as nodular amorphous precipitates which were identified as CaP, or bone nodules.
The group attributed these differences in hMSC behavior to the different soluble factor release rates exhibited by the two materials. Psw has a much higher initial dissolution rate than wol, presumably because its highly strained structure makes it more susceptible to hydrolysis. This resulted in a large initial release of silicon atoms, which caused the initial low cell viability, and calcium atoms, which caused increased calcite and calcium phosphate formation.
Overall, the study proved that psw is more osteoinductive than wol, thereby showing that crystal structure can play an enormous role in determining the osteoconductive and osteoinductive properties of a bioceramic. The collected data mark a promising step forward in the creation of a model that can successfully predict osteoinductivity based on a bioceramic’s inherent material properties.
Crystal structures of CaSiO3 polymorphs control growth and osteogenic differentiation of human mesenchymal stem cells on bioceramic surfaces
Nianli Zhang, James A. Molenda, Steven Mankoci, Xianfeng Zhou, William L. Murphy and Nita Sahai
Biomater. Sci., 2013, 1, 1101-1110 DOI: 10.1039/C3BM60034C
Ellen Tworkoski is a guest web-writer for Biomaterials Science. She is currently a second year Ph.D. student in the biomedical engineering department at Northwestern University (Evanston, IL, USA).
Follow the latest journal news on Twitter @BioMaterSci or go to our Facebook page.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents alert.