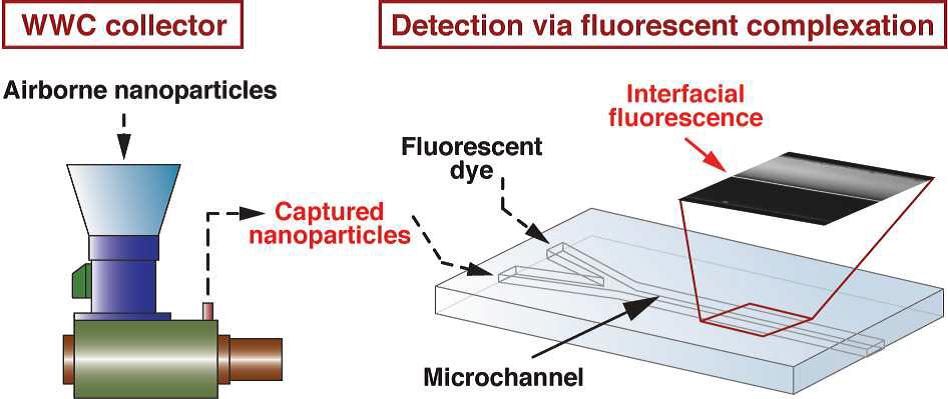
Fluorescence complexation could hold the key to more detailed monitoring of airborne nanomaterials. Researchers at Texas A&M University, USA describe the successful development of a new method of simultaneous online quantification and characterization.
The increased manufacture and use of nanomaterials has led to increased concerns about their associated health risks, particularly in the occupational setting. One of the main barriers to addressing uncertainties in this field is a poor understanding of personal exposure levels. There is currently a lack of sufficient dynamic data regarding the main exposure routes for airborne nanomaterials so appropriate exposure guidelines, profiles and models cannot be established.
It is important, therefore to establish effective means of measuring the levels and chemical/physical properties of nanomaterials in real-time. Preferred traditional means of nanomaterial analysis e.g. mass spectrometry (MS) and electron microscopy (EM) cannot be easily adapted to use in an online capacity. This means exposure analysis is generally slow and can only be carried out for relatively short, unrepresentative time-frames.
In this study Fanxu Meng and co-workers introduce and demonstrate an integrated methodology that allows continuous online monitoring of the levels and characterization of airborne nanomaterials. This method combines ultra high flow sampling with a sensitive fluorescence-based detection system.
The sampling system comprised a modified wetted wall cyclone (WWC) collector which has an ultra high flow rate (>1000 L min-1) combined with a continuous flow microfluidic network allowing online detection capability. The detection system utilized florescence generated by the combination of a suspension of collected nanoparticles and a tracer dye solution. The resulting dye-nanoparticle complex produces an intense and easily detectible fluorescent signature, dependent on the quantity and the physical/chemical properties of the collected nanomaterials.
The integrated system was tested using prepared suspensions of Al2O3. A scanning mobility particle sizer (SMPS) was used to quantify the concentration and size distribution of nanoparticles inside the test chamber. It was shown that florescence displays a characteristic pattern, dependent on the concentration but also the size and particle surface area of the nanomaterials. A linear correlation between florescence intensity and airborne concentration of Al2O3 at concentrations up to 1.0-1.2 wt% at flow rates of 0.2 and 0.02 mL min-1 was observed.
This work provides a platform for continuous measurement of airborne nanomaterials. Simultaneous sampling and compound characterization can enable better time-resolved assessment of the transport and fate of released nanomaterials and identification of hazardous releases. The method described is capable of sampling a broad range of air volumes (representative of the work place environment) and allows online detection and analysis. The authors highlight potential further innovations for this work, possibly leading to more detailed exposure profiles for nanomaterials; establishing fluorescence “fingerprints” for a range of different nanoparticles; and analysis of biological material.
Download the full article for free* today!
Localized Fluorescent Complexation Enables Rapid Monitoring of Airborne Nanoparticles
Fanxu Meng, Maria D. King, Yassin A. Hassan, and Victor M. Ugaz
DOI: 10.1039/C4EN00017J
*Access is free through a registered RSC account – click here to register