The sites of underground repositories for radioactive waste need to be selected, designed and built adequately. This requires an in-depth understanding of the geochemical processes governing the release and transport of radionuclides from the waste to the surrounding environment. In this study published in Environmental Science: Processes & Impacts, researchers describe a technique that will improve our knowledge of potential leaching of radionuclides in these environments.
Many countries around the world now meet a substantial fraction of their energy demand through nuclear power. A key environmental issue, therefore facing these nations, is how to ensure the safe and responsible disposal of radioactive wastes. The International Atomic Energy Agency outlines a number of different potential methods for disposing of radioactive wastes and discusses the approach of different nations.
Careful burial in well-engineered ‘repositories’ at various depths below the land surface – so-called ‘geological disposal’ – is now the preferred option for the final storage of nuclear waste for most countries with advanced nuclear programmes, including the UK, Canada, Finland, France, Sweden, Switzerland, Japan and the USA. Indeed, a 2004 European Commission Report on radioactive wastes states that:
“Burial at several hundreds of metres depth in stable rock environments is the option for disposal of the most hazardous radioactive wastes because it will provide permanent safety – not just for ourselves, but for future times very much longer than the whole of past human history.”
However, in order to ensure that this statement is true, it is essential to assess to what extent radionuclides could be released to the environment. Therefore, it is of great importance to understand how long-lived radionuclides (such as 79Se, 129I, 14C or 36Cl) are chemically bound in the radioactive waste matrix. The challenge for researchers and practitioners is to provide reliable safety assessments for such nuclear waste repository sites that provide reliable long-term predictions on the release of radionuclides in waste repositories as the waste undergoes geochemical transformations in ground waters.
Radiocative wastes are typically a highly heterogeneous material made up of the fuel matrix with 3–6% fission products and minor actinides dispersed among different phases. Long-lived isotopes like 79Se, 135Cs, 129I and 36Cl are of interest because they are easily soluble in water and sorb only weakly on mineral surfaces, implying that, once dissolved, under repository conditions they will migrate through the sub-surface environment very rapidly. These compounds are therefore major contributors to the overall radiological dose calculated in risk assessments of nuclear waste repositories.
The properties and behaviour of radionuclides like 79Se in nuclear wastes are not well understood due to the technical difficulty of obtaining sound experimental data on such highly radioactive materials. This insufficient knowledge is usually compensated by conservatism in the choice of parameter values for safety assessment calculations. For example, it has previously been assumed that a significant fraction of 79Se is rapidly released from the spent fuel waste on contact with aqueous solutions and is highly mobile. This is due to the observation that selenium has an appreciable volatility under reactor operation conditions and the high solubility of oxidized Se species in water.
However, recent experiments have indicated that less than 1% of the Se in a geological disposal repository is released to aqueous solution after 1 year leaching, suggesting only a small fraction is actually leachable. This demonstrates the need to further investigate the geochemical nature and behaviour of long-lived radionuclides such as 79Se in radioactive wastes and the interaction of these isotopes with spent UO2 fuel.
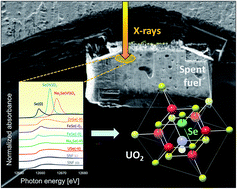
This work is the result of a collaboration between Swiss, Swedish, French and American research institutes, investigating radionuclide release of 79Se from radioactive waste in a deep water-saturated repository. In the study, X-ray Absorption Near Edge Spectroscopy (XANES) measurements were made on samples from the Leibstadt Boiling Water Reactor in Switzerland.
Their results offer a mechanistic explanation why Se appears to be much less soluble in short-term aqueous leaching experiments, compared to other radionuclides like I and Ce. It was shown that these results were corroborated by a simple thermodynamic analysis, showing that selenide is the stable form of Se under reactor operation conditions.
This study provides a technique that helps improve our understanding of the geochemical transformation and transport of radioactive nuclides in wastes disposed in geological formations. Investigations like this are required to reduce conservatism and improve reliability in carrying out safety assessment calculations. This work is therefore integral to the future selection and design of potential nuclear waste repository sites.
To read more about this research, download a copy of the manuscript for free* by clicking the link below.
Characterization of selenium in UO2 spent nuclear fuel by micro X-ray absorption spectroscopy and its thermodynamic stability
E. Curti, A. Puranen, D. Grolimund, D. Jädernas,D. Sheptyakov and A. Mesbah
Environ. Sci.: Processes Impacts, 2015,17, 1760-1768
DOI: 10.1039/C5EM00275C
—————-
Ian Keyte is currently a Science Policy Intern at the Royal Society. He previously gained a PhD at the University of Birmingham investigating atmospheric pollution, and has a BSc in Environmental Chemistry from Lancaster University.
—————-
* Access is free until 24/11/2015 through a registered RSC account.