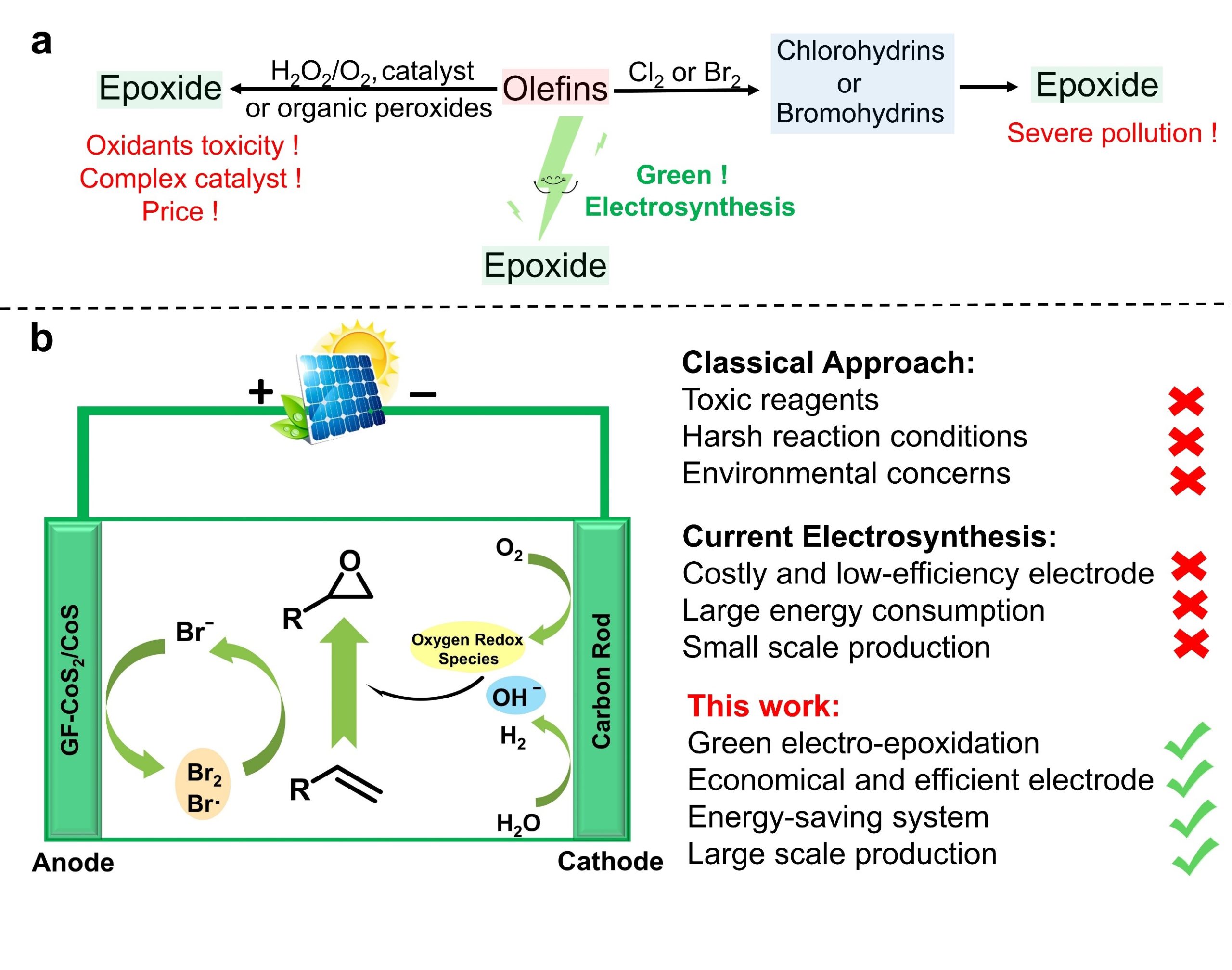
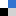Among various oxidation reactions, olefin epoxidation is undoubtedly one of the most studied organic conversion reactions. The target product epoxide is amongst extensively used chemicals and has important applications in the synthesis of polymers, food additives and drugs. Therefore, efficient synthesis of epoxides holds a significant place for academic and industrial research perspectives. Traditional olefin epoxidation methods generally require toxic and harmful, high-priced oxidants with harsh reaction conditions and difficult separation. The most commonly used process for the industrial synthesis of epoxides is the halogenated alcohol (Br, Cl) method (HALCON process). However, this process produces a large amount of chlorine (bromine) wastewater during the production process, causing serious pollution to the environment. The epoxidation of olefins by electrochemical oxidation of bromide ions is an emerging and promising strategy (Scheme 1). The choice of the electrode is very important for electro-epoxidation process. The common electrodes currently being used in the scientific community are platinum electrodes and carbon electrodes, however they all have certain limitations. Although the platinum electrode has good catalytic activity for the oxidation of bromide ions, but it can be easily corroded by bromide ions, which affect its catalytic activity and causes the waste of precious metal. In addition, carbon electrodes are also known to possess low catalytic activity. Therefore, the development of an economical and efficient electrode that could promote the oxidation of bromide ions for the facile epoxidation of olefins still needs to be endeavoured.
Recently, Zai’s group has reported a strategy for the electrochemical, efficient and green synthesis of styrene epoxide. First, the team synthesized a series of metal sulfide electrodes by solvothermal method, and analysed the catalytic activity of metal sulfide electrodes for bromide ion oxidation via linear sweep voltammetry (LSV). The team noticed that cobalt-based sulfides, especially heterostructure CoS2/CoS, can effectively promote the oxidation of bromide ions on the anode. Subsequently, the electrochemical epoxidation of styrene was carried out with metal sulfides as the anode and carbon rod as the cathode, and a series of optimizations were carried out for the optimal reaction solvent, time and temperature. Under the optimal reaction conditions, the platinum electrode (Pt) displayed a selectivity of 55% for styrene epoxide. This may be due to the surface corrosion of platinum electrode by bromide ions during the reaction and the other reason could be the smaller electrochemical surface area (ECSA) of the platinum electrode. Moreover, the metal sulfide electrodes Cu7S4, CdS, MnS, FeS2, NiS2 have 73%, 76%, 78%, 80%, and 81% selectivity to Styrene epoxide, respectively. It is worth noting that the selectivity of CoS and CoS electrodes towards styrene epoxide reached 88% and 90%, respectively. When CoS and CoS2 were combined to form heterojunction CoS2/CoS, Highly selective synthesis of styrene epoxide (97%) was attained with GF-CoS2/CoS as anode and carbon rod as cathode. The applied voltage was reduced to 4-5 V at 30 mA cm-2 compared to 7.8-9.3 V on Pt cathode, which effectively reduces the energy consumption of the reaction. Subsequently, the team conducted in-depth studies on the mechanism (Scheme 2) involved in the electrochemical epoxidation of styrene by conducting experiments in different electrolytes, different atmospheres, different reaction cells (single, H-type), and different free radical quenchers (Figure 1).
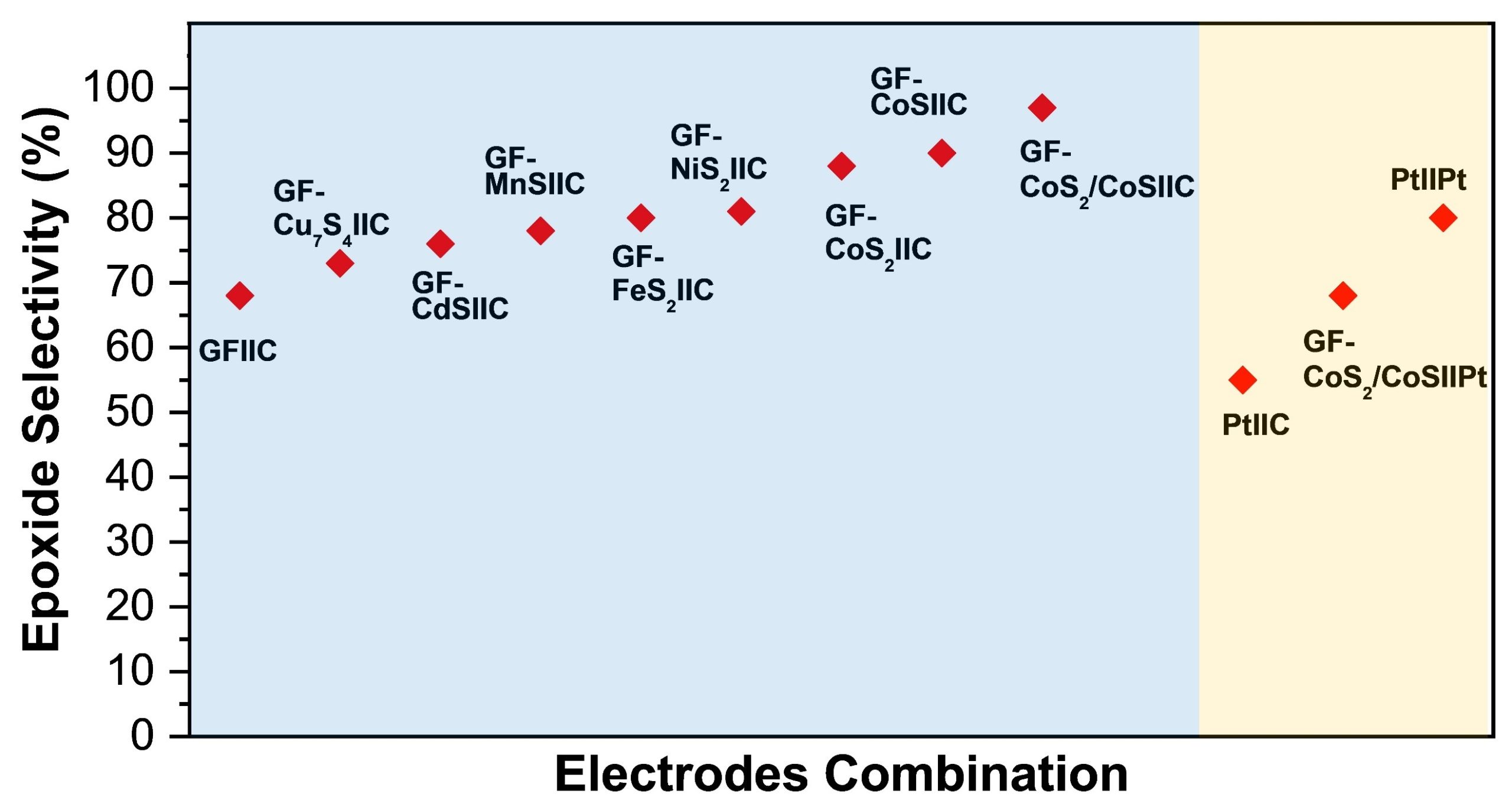
Figure 1 Optimization of electrode systems. Blue zone: Metal sulfide-based electrodes as anode. Yellow zone: Pt used as anode or cathode.
The mechanistic investigations inferred the generation of oxygen redox species (such as OH•, H2O2, O2•¯) besides the olefin-based radicals and bromine radical, facilitating the epoxide formation. The synergistic cooperation between the bromine redox species and oxygen redox species (generated on graphite rod cathode) can establish a benign electro-epoxidation system for olefin substrates in an ambient environment (Scheme 3). The group demonstrated that the proposed strategy could also be applied to other olefins. As this strategy is simple, sustainable, and economical so, it could be of great significance for technical aspects.
Corresponding author:
Jiantao Zai is associate professor in the school of chemistry and chemical engineering in Shanghai Jiaotong University. He is engaged in the design, synthesis and performance research of inorganic solid materials, especially micro-nano, multi-component functional materials. He has published 106 papers in journals such as Nat Commun, Adv Energy Mater, Nano Energy, etc. The papers have been cited 4559 times, and the H index is 40. In 2015, he won the first prize of Shanghai Natural Science (No.5), and was selected as Shanghai in 2018. Youth Science and Technology Venus (Class A).