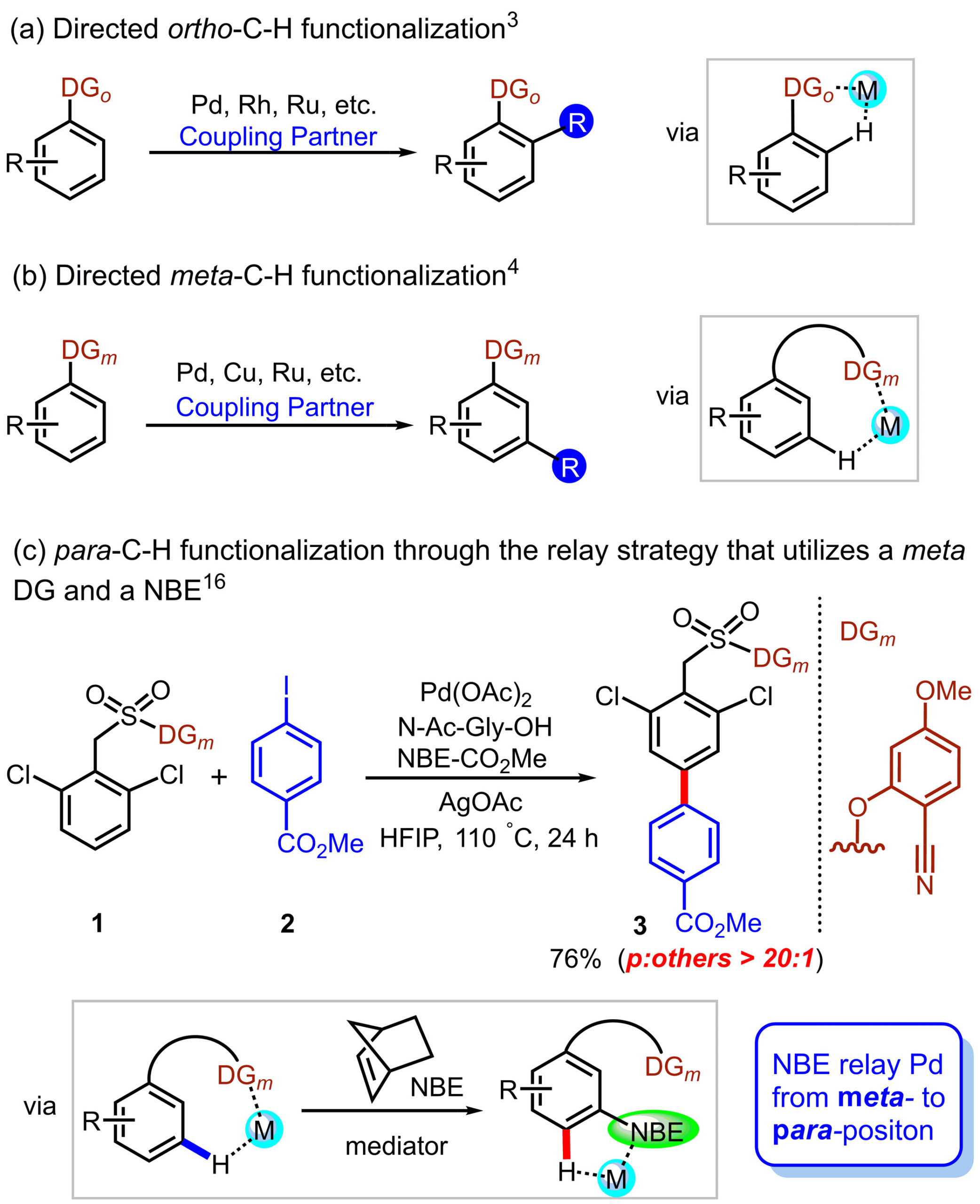
Selective C-H aromatization of aromatic compounds has long been a challenging issue. Directing group strategies can only make the ortho- or meta-sites of the aromatic compounds activate in past years. Recently, an elegant approach was reported to successfully achieved the para-arylation of arene by means of a cooperative action of directing group with norbornene relay (Scheme 1). This strategy first implements meta-C-H activation through directing group guidance, subsequently, the transient mediator norbornene relay Pd to the para-position to achieve precise siteselectivity. Understanding this novel relay mechanism is of great significance for achieving more extensive and accurate site selection.
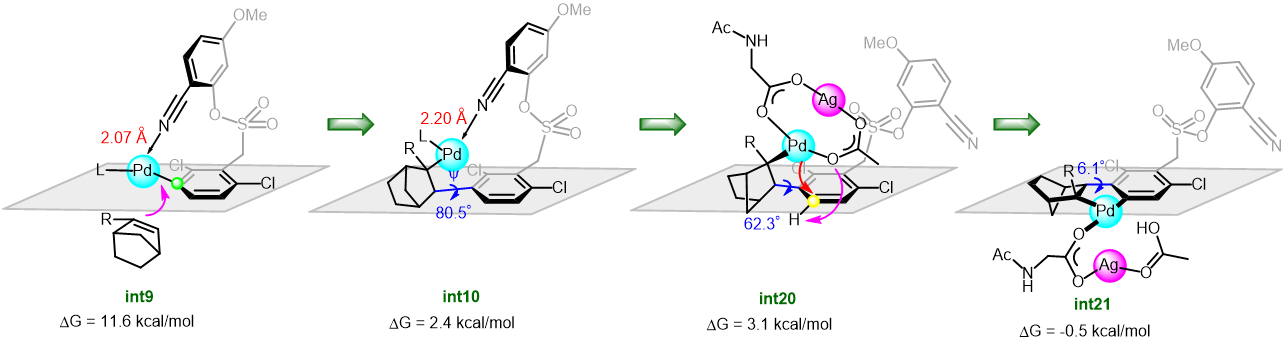
Dezhan Chen and collaborators explored in detail the mechanism of the arylation of arenes by means of norbornene relay palladation through meta- to para-selectivity using DFT calculations. The results revealed that the reaction was initiated by a [mono N-protected amino acid ligand-Pd] complex to activate firstly the meta-C-H guided by the directing group. The para-arylation was subsequently achieved by NBE relay palladation from meta- to para-position (Figure 1). Significantly, the palladium/norbornene cooperative relay was realized by a bimetallic Pd-Ag complex. The authors demonstrated that the Pd-Ag bimetallic complex play a significant stabilization role in secondary para-C-H activation rather than the intuitive tether length (Figure 2).
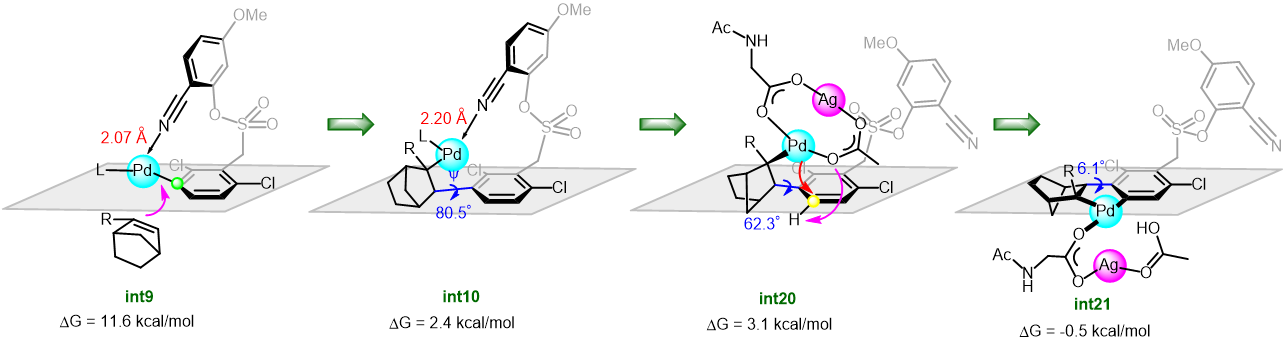
Figure 2. Free energy profiles for the para-C-H activation catalyzed by monomeric Pd and heterodimeric Pd-Ag.
The calculated energy profiles of the NBE relay Pd through meta- to para-position to produce para-arylated product is summarized in Figure 3a (black pathway). The author further optimized the energy profiles of NBE relay palladation through para- to meta-position (blue pathway), while this pathway was kinetically unfavorable. The calculation results shown that directing group assisted primary C-H activation was the rate-determining step for the overall catalytic cycle, also the key step of determining siteselectivity. The primary meta-activation was favorable in energy due to less ring strain in the cyclic nitrile-coordinated C-H transition states in meta-position. As illustrated by the Gibbs energy profile in Figure 3b, norbornene insertion, as the key step to achieve the expected Pd relay, can take place spontaneously with exothermic of 7.1 kcal/mol, which can compensates for the energy needed to cross the barrier. It follows that the palladium relay process is driven thermodynamically to achieve the activation from meta- to para-position.
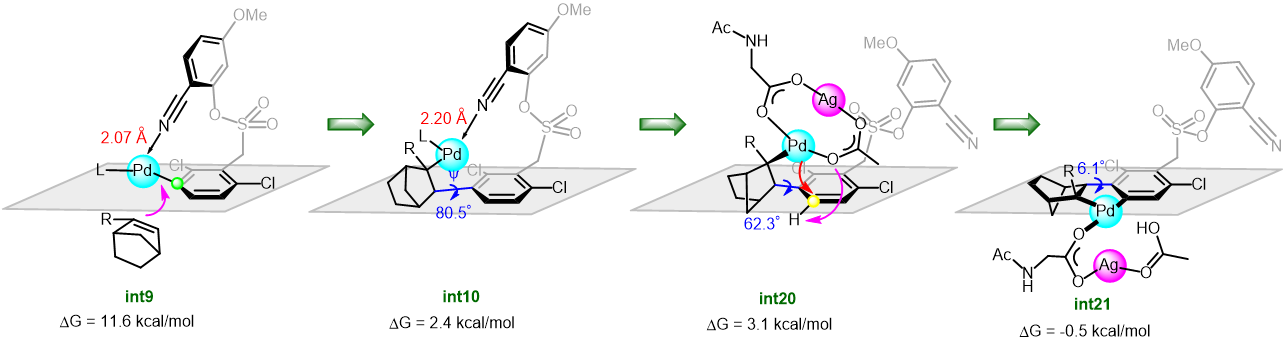
Figure 3. (a) Free energy profiles of the full catalytic cycle of two pathways. (b) Free energy profiles for the major and minor pathway based on Curtin-Hammett scenario.
The perfect cooperation of a remote directing group and a transient mediator NBE through the alternating association with the Pd center achieved the active site relay through meta- to para-position. The present results provided a reasonable insight into the para-C-H arylation by Pd/MPAA/NBE cooperative catalysis in conjunction with a precise directing group and Ag(Ⅰ) additive and would have implications for understanding C-H functionalization chemistry by norbornene relay palladation.
Dezhan Chen is professor in College of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University (China). He received his master’s degree in Department of Chemistry of Shandong University in 1989. He studied in UCLA at Houk group as a visiting scholar in 1996-1997. The research interest of Professor Chen is the exploration of theoretical mechanism of chemical reaction and its thermodynamic and kinetic processes.