Helical structures are ubiquitous in nature. DNA or right-handed α-helix of proteins exemplify the sophistication of natural, helical structures whose chirality stems from an efficient transfer of asymmetry from the constitutive L-amino acids or D-carbohydrates. Inspired by these functional, helical structures, a plethora of examples of man-made helical architectures by using macromolecules or small molecules have been reported in literature. The helical structures constituted by small molecules are especially interesting since the generation of such helices involves an organized arrangement of assembled or self-assembled monomeric units that, very often, possess inherent elements of asymmetry. On the other hand, supramolecular polymers, macromolecular species constituted by the non-covalent interaction of monomeric units, have emerged as very useful benchmark to construct and investigate helical structures. The decoration of these monomeric units with elements of asymmetry usually provokes the efficient transfer of asymmetry from the molecular to the supramolecular level, thus, generating helical supramolecular polymers.
However, to the best of our knowledge, there are no studies on the optimal distance for point chirality to afford an efficient transfer of asymmetry that results in the formation of helical supramolecular polymers. Recently, the group of Prof. Luis Sánchez, at the Department of Organic Chemistry (Universidad Complutense de Madrid, Spain) has reported on the synthesis of a series of self-assembling N-annulated perylenes 1-4 (Figure 1a) endowed with chiral, peripheral side chains located at increasing distance from the aromatic moiety. The p-surface of the N-annulated perylene and the presence of the amide functional groups favour the self-assembly of 1-4 by π -stacking of the aromatic units and the formation of an array of H-bonding interactions, respectively. The presence of the trialkoxybenzamide moiety bridged to the N-annulated perylene unit by a linear spacer of variable number of methylene units allows the formation of an intramolecularly H-bonded pseudocycle (Figure 1b) that, in the case of compounds 1-3, retards their cooperative supramolecular polymerization. This is not the case of compound 4 in which the separation between these two structural units makes difficult the efficient formation of the 10-membered H-bonded pseudocycle.
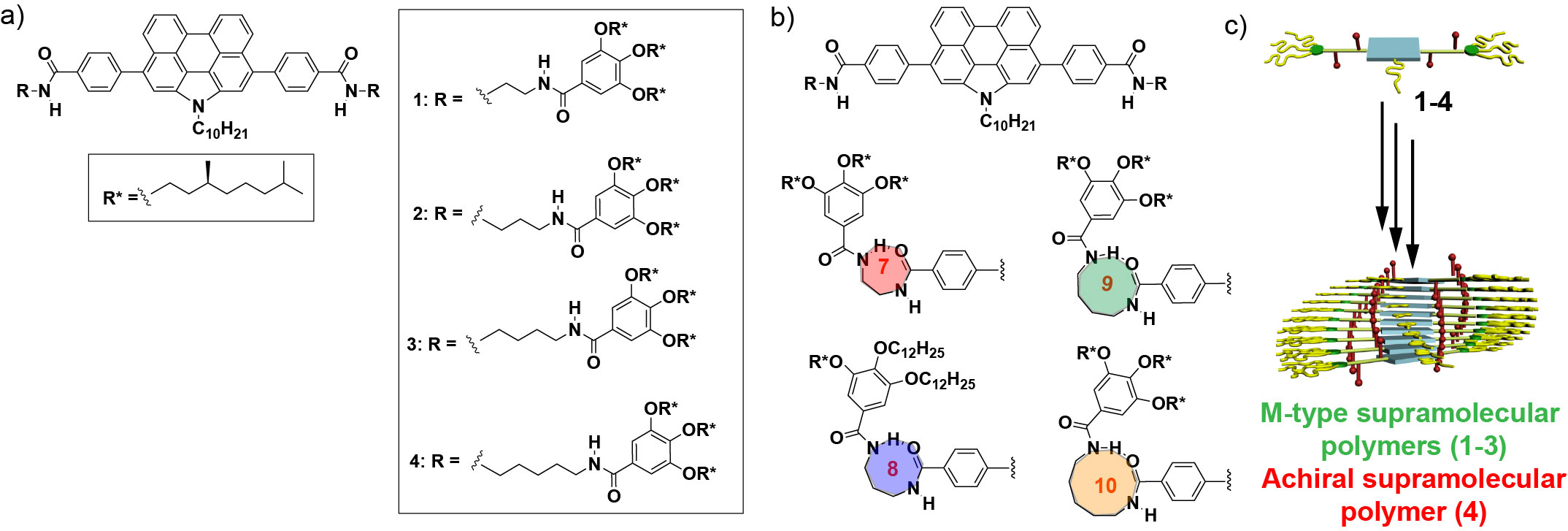
Figure 1. Chemical structure of the N-annulated perylenetetracarboxamides 1–4 showing the open arms (a) and the metastable 7–10-membered pseudocycles (b); (c) Schematic representation of the cooperative supramolecular polymerization of 1-4 that yields M-type helical aggregates for 1-3 but achiral aggregates for 4.
The increasing separation between the peripheral side chains in compounds 1-4 provokes a clear depletion of the dichroic response that indicates the reduction on the ability for transferring asymmetry from compound 1 to compound 4, in which no dichroic response is registered (Figure 2a and 2b). However, the strong trend of compound 4 to bundle results in a clear anisotropic organization of the supramolecular fibers formed upon its self-assembly that affords a strong linear dichroism effect (Figure 2c)
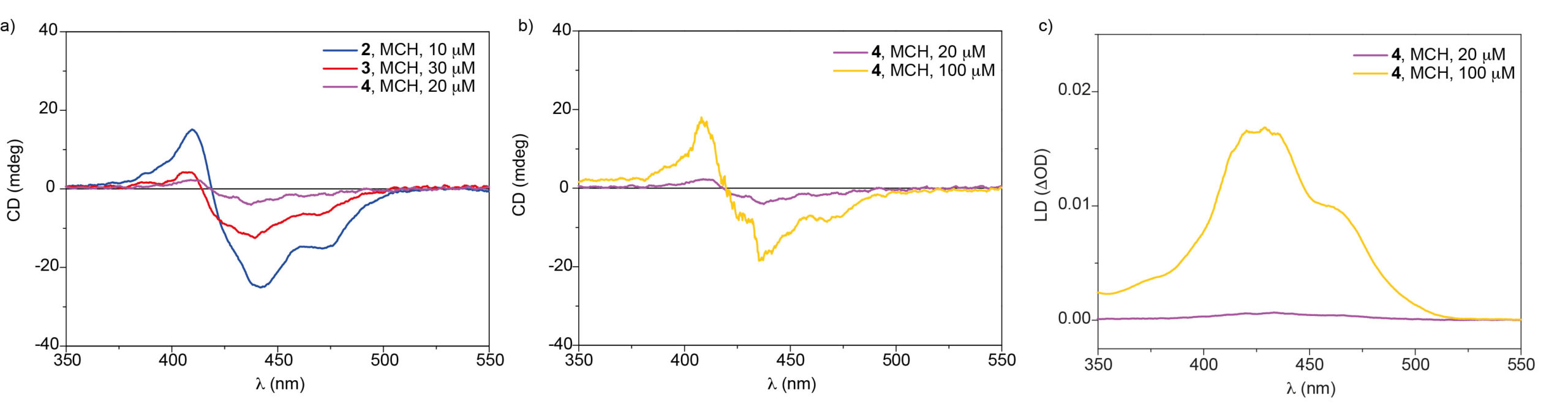
Figure 2. a) CD spectra of compounds 2-4 in MCH at 25 ºC; CD (b) and LD (c) spectra of 4 in MCH at different concentrations.
The results presented herein contribute to establish a structure-function relationship in the generation of helical supramolecular structures and to shed light on the intricated process of the origin of natural homochirality.
Org. Chem. Front., 2021, Advance Article
https://doi.org/10.1039/D1QO00837D
Luis Sánchez Martín (ORCID: 0000-0001-7867-8522) is Full Professor in the Department of Organic Chemistry at the Universidad Complutense de Madrid (Spain). He received his PhD in Chemistry at the Faculty of Chemical Sciences in 1997. From 1999 to 2000 he did a postdoctoral stay at Rijksuniversiteit Groningen (The Netherlands). The research interest of Prof. Luis Sánchez is the investigation of the supramolecular polymerization of electroactive monomeric units and the studies of transfer and amplification of asymmetry from the molecular to the supramolecular level to yield helical structures. He is coauthor of more than 135 articles indexed by SCI, cited more than 10000 times and with an index h = 48.