Mechanically interlocked molecules (MIMs) have received an increasing attention from the scientific community during the last decades. Among their potential applications in numerous fields, the design of molecular machinery based on MIM is one of the most prominent. The presence of at least two mechanically bonded components in the MIM´s backbone allows these systems to undergo a variety of large-amplitude internal dynamics, making them ideal candidates to be included in artificial machines.
Lately, mechanized systems, mainly [2]rotaxanes, have been found as interesting molecules to be used as ligands in metal-mediated transformations or as organocatalysts, both in achiral and chiral processes. Due to the internal translational motions, remarkable examples of switchable catalysts have been described. The catalytically active site present on the thread can be exposed or concealed by the bulky macrocycle by action of an external stimulus, altering the catalytic activity of the systems (Catalysis ON or OFF, Figure 1). Moreover, the orthogonal disposition of the two components, creating dynamic enzyme-like 3D cavities, can be advantageous for the control of the reaction outcomes, highly important in asymmetric processes. Consequently, catalytically active threads are generally more reactive (and less selective) than their corresponding rotaxanes, in which the active site is hindered by the ring.
Recently, the researchers Alberto Martinez-Cuezva, Jose Berna and members of the Synthetic Organic Chemistry Lab of the University of Murcia and the group of Marcos A. P. Martins(NUQUIMHE) from the Universidade Federal de Santa María (Brazil) have found that interlocked organocatalysts can be more active than their corresponding threads (Figure 2a). The authors synthesized a series of one- and two binding-site threads 1 and their corresponding hydrogen-bonded rotaxanes 2 by following a clipping methodology. These systems have succinamides as binding sites and a secondary amine as the active site, able to catalyze iminium-type transformations. All the systems were evaluated as catalysts in the Michael addition of acetylacetone to crotonaldehyde, following the conversion over time towards the formation of the Michael adduct (Figure 2b). Remarkably, the two binding-site systems were more active to the one binding-site analogs, and more importantly, the interlocked catalysts were more efficient than the corresponding free threads. The presence of the bulky polyamide macrocycle is able to activate the electrophile (aldehyde) towards a nucleophilic attack of the amine. Moreover, the iminium intermediate is stabilized by the polyamide ring.
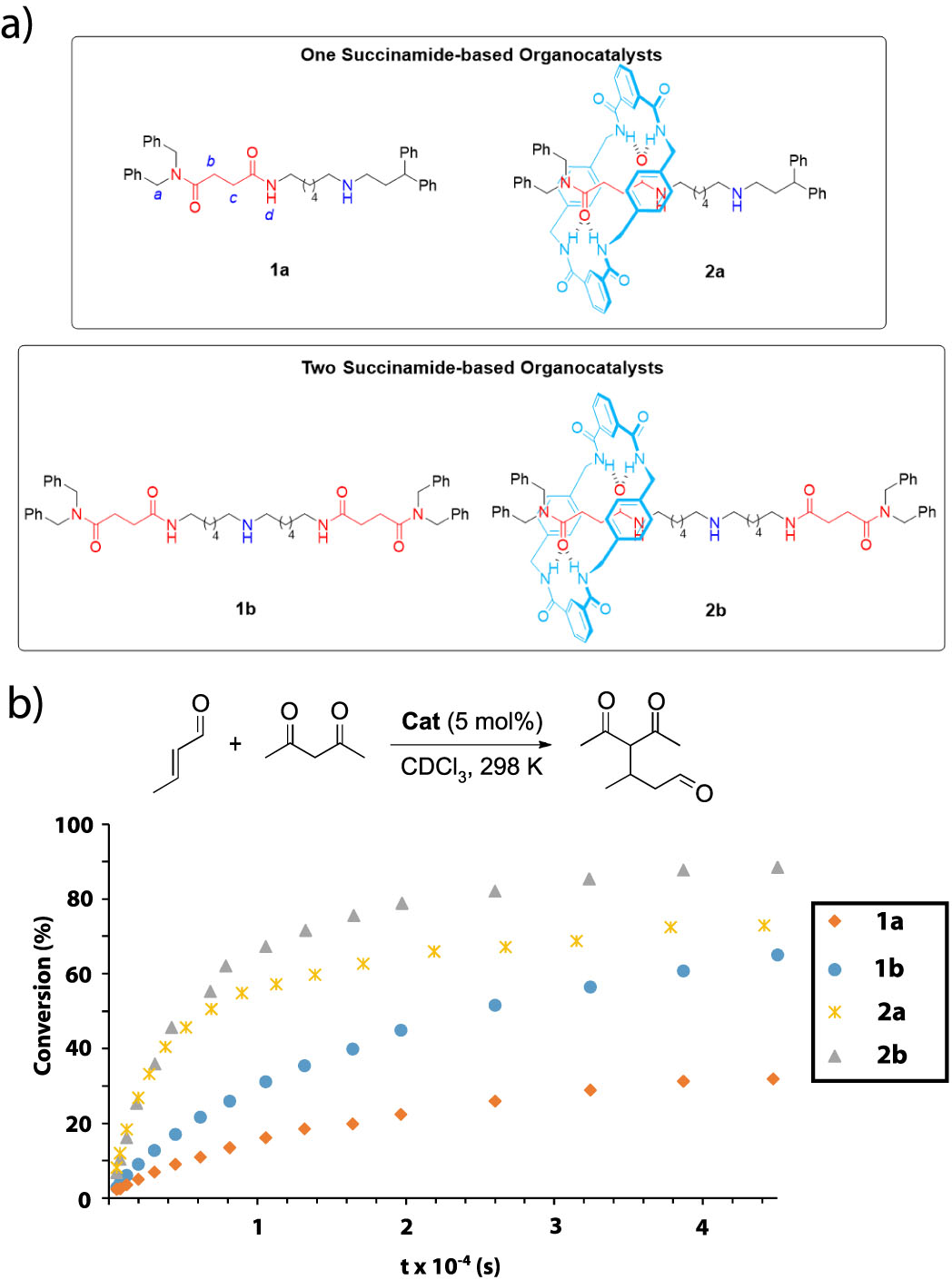
Figure 2. a) Organocatalysts evaluated in this study; b) Conversion of the Michael addition of acetylacetone to crotonaldehyde over time catalyzed by the threads and their corresponding [2]rotaxanes.