π-Conjugated macrocycles are molecules with unique properties that are increasingly exploited for applications. Their study began in the early 1960s and many π-conjugated macrocycles have been synthesized since. However, only recently the field has moved towards making use of the unique properties of these cyclic molecules for applications. π-Conjugated macrocycles are now being investigated in organic solar cells, photodetectors, field-effect transistors, light-emitting diodes, and battery electrodes. They can also be used in bioimaging, as templates for the growth of carbon nanotubes, and as molecular nanoreactors.

Figure 1. (a) Reversible two-electron reduction of a π-conjugated macrocycle for charge storage in organic battery electrodes. (b) Visualization of chemical shielding tensors (VIST) helping to rationalize the optoelectronic properties.
Despite this recent interest in π-conjugated macrocycles, there are only a small number of experimental studies that investigate how the properties of π-conjugated macrocycles evolve with systematic structural changes. Recently, a team around Florian Glöcklhofer has reported such a systematic experimental study and combined it with an in-depth computational analysis. The study reveals the central role of local and global aromaticity for rationalizing the optoelectronic properties of the macrocycles. A recently developed computational method for the visualization of chemical shielding tensors (VIST) was applied to provide unique insight into local and global ring currents occurring in different planes along the macrocycles.
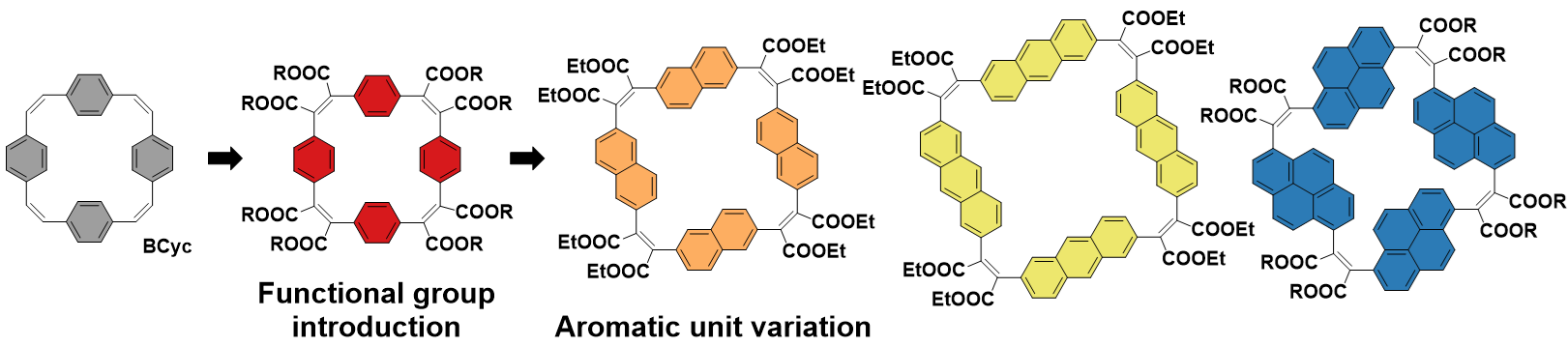
Figure 2. Functional group introduction and aromatic unit variation in a set of π-conjugated macrocycles.
The study makes a significant contribution to the development of structure–property relationships and molecular design guidelines and will help to understand, rationalize, and predict the properties of other π-conjugated macrocycles. Furthermore, it shows that cyclophanetetraenes, the investigated class of macrocycles, provide versatile scaffolds for applications due to their unusual properties along with their high tunability. Their remarkable optoelectronic properties, in particular their large Stokes shifts and the accessibility of a variety of charged states, were traced back to their formal ground state antiaromaticity along with high structural flexibility.
Rimmele, M.; Nogala, W.; Seif-Eddine, M.; Roessler, M. M.; Heeney, M.; Plasser, F.; Glöcklhofer, F., Functional group introduction and aromatic unit variation in a set of π-conjugated macrocycles: revealing the central role of local and global aromaticity, Organic Chemistry Frontiers 2021, Advance Article. https://doi.org/10.1039/D1QO00901J
Author’s Biography
Florian Glöcklhofer is an Erwin Schrödinger Fellow in the Department of Chemistry at Imperial College London. He received his PhD in 2017 at TU Wien (Vienna) for developing a reaction for the conversion of quinones into cyanated aromatic compounds. His research in the field of π-conjugated organic compounds focuses on the design and synthesis of π-conjugated macrocycles for battery electrodes and organic electronics and on the development of new synthetic approaches to aromatic organic compounds.
https://www.gloecklhofer-research.com/











