To combat global warming and the climate issue, clean and sustainable energy alternatives to fossil fuels must be developed. Due to the high energy density (142 kJ/mol) and lack of environmental effects, hydrogen is regarded as a clean fuel. Besides this optimistic view, there is still an urgent need for a green, sustainable, and efficient technique for mass production of hydrogen. Electrochemical water splitting is the only method to produce enormous quantities of highly pure hydrogen under favourable conditions, with only a by-product of water. The two half-reactions involved in electrochemical water splitting are the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). The nominal breakdown voltage for splitting water is 1.23 V in theory, but in real electrolytic experiment, an extra energy (overpotential) is required to overcome the kinetic barrier and produce hydrogen at a fast enough rate. Overpotential causes energy consumption to rise, which reduces electrochemical water splitting efficiency and a system’s capacity to compete economically. So, the use of electrocatalyst would increase the current density at a given voltage by minimizing the overpotential and further catalysing the electrochemical processes. Hence, a powerful electrocatalyst with high catalytic performance and good static stability is essential for the commercially sustainable production of hydrogen on a wide scale using an electrochemical process. Apart from noble metals, an earth-abundant non-noble metal-based electrocatalyst has been developed significantly over the past few years to minimize the practical cost of the electrocatalyst.
Recently, researchers at the Materials Chemistry Laboratory for Energy, Environment and Catalysis at CSIR-Central Electrochemical Research Institute (CECRI), India has constructed a review on Metal-Organic Framework (MOFs) based materials as electrocatalyst for water splitting especially focusing on OER with brief description over the fundamental and structural aspects (Fig. 1). MOF will be used as a viable contender for many applications, including catalysis, sensing, luminescence, drug administration and imaging, adsorption and separation, gas and energy storage, due to the cage-like structure. The primary benefits of these MOFs include flexible and tunable chemical functions with a variety of organic linkers, tunable shape, high specific surface area, and configurable pore size. Again in this review, they briefly deliberated the most recent advancements in MOF-based electrocatalyst in terms of design and fabrication, characterisation, and catalytic mechanism towards water splitting reaction. In addition, this review also emphasized the challenges and potential of employing a MOF-based materials for water splitting.
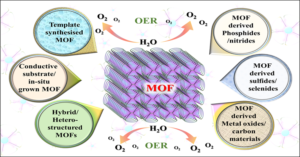
Fig.1. Schematic representation of the recent advancement of MOF based materials towards electrocatalytic oxygen evolution reaction (OER).
Corresponding Author:
Dr. Subrata Kundu received his Ph.D from the Indian Institute of Technology (IIT), Kharagpur, India in early 2005. Then he moved to University of Nebraska, Lincoln, USA and later to Texas A &M University, College station, Texas, USA as a post-doc fellow (from 2005 to 2010). He is currently working as a Principal Scientist at CSIR-CECRI, Karaikudi, India. Dr. Kundu is serving as an associate editor of prestigious ‘Journal of Materials Chemistry A’ and ‘Materials Advances‘ from RSC publishers since 2022. Dr. Kundu and his co-workers are working in the forefront area of Material Sciences with emphasizes on energy, environment, catalysis and electrocatalysis applications.