Photochromic dyes are a specific class of molecules that can undergo reversible transformations under exposure to light between isomers that possess different optical properties. This peculiar feature makes them appealing for a myriad of applications, ranging from bioimaging, data storage, sensing or optical lenses.
Very recently, they have been employed in photovoltaics. Few photochromic dyes have been investigated as photosensitizers in dye-sensitized solar cells (DSSCs) and the only photochromes that have demonstrated a fully reversible photochromic process with polychromatic light once embedded in solar cells are diphenyl-naphthopyran derivatives, as reported by the group of Renaud Demadrille of CEA Grenoble in France and collaborators of the University Pablo de Olavide in Spain. Using this class of dyes, they demonstrated the fabrication of semi-transparent smart photovoltaic devices capable to self-adapt their transparency to the ambient light. Recently, they have designed and synthesized a series of spiro-indoline naphthoxazine (SINO) and spiro-indoline naphthopyran (NIPS) with a donor-photochrome-acceptor (D-p-A) chemical structure for their use in DSSCs.

Figure 1. General structure of the new dyes and the interconversion reaction between their close (CF), merocyanine (MC) and protonated merocyanine forms (MCH).
The interconversion process and optical properties of the two SINO and two NIPS dyes synthesized in this work have been proven more complex to unravel than the ones of diphenyl-naphthopyrans, as they are not only photochromic but also acidochromic. The interactions of the molecules with both stimuli, i.e. H+ and light, have been studied separately or simultaneously, in solution or after grafting onto the surface of TiO2. A positive photochromism in solution characterized by extremely fast thermal discoloration kinetics has been observed for some of the compounds. All the dyes demonstrated acidochromic properties, but in the presence of acid, only NIPS derivatives showed a negative photochromism, i.e. a fast bleaching under illumination.
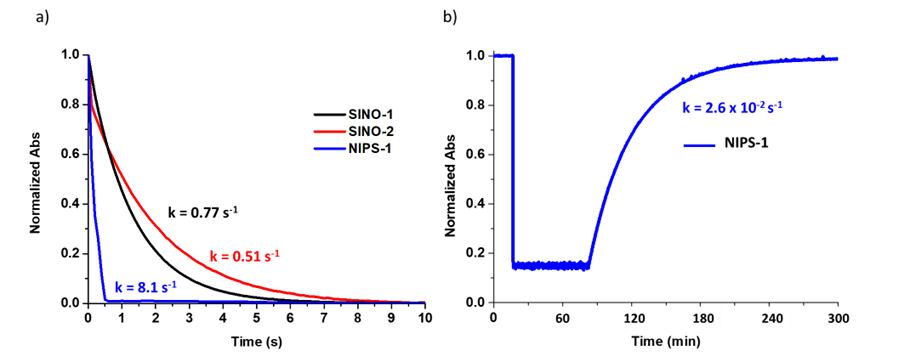
Figure 2. (a) Normalized discoloration curves of SINO-1, SINO-2 and NIPS-1 and (b) coloration curve of NIPS-1 after the addition of HCl and bleaching with light (2 x 10-5 solutions at 25 °C, irradiation source: 200-600 nm/200 W xenon lamp).
After unraveling the photochromic and acidochromic properties of the new molecules, they were employed in the fabrication of dye-sensitized solar cells. The choice of the electrolyte was found to be critical, due to the pH-driven optical behavior of the dyes. If a low-pH electrolyte is used, a non-photochromic protonated open form was mainly produced, which was mostly avoided in the case of a neutral electrolyte. This work highlights that the photochromic properties of the dyes can be conserved when going from the solution to the devices for some of the dyes.

Figure 3.(a) J-V curves (dashed lines recorded in the dark, solid lines recorded under illumination) of opaque NIPS-2-based DSSCs using our acidic homemade electrolyte (black) and Iodolyte (red) and (b) transmittance spectrum of the transparent device together with a picture of the NIPS-2/Iodolyte device (standard irradiation conditions AM 1.5 G, 1000 W m−2; 25 °C; active area= 0.36 cm2).
This study is the first to investigate in detail the complex relationships between photochromic, acidochromic and photovoltaic properties for these classes of dyes. The structure-property relationships established will undoubtedly be useful for the development of new photochromic compounds with optimized optoelectronic properties for applications in various fields, including photovoltaics.
Corresponding authors:
José María Andrés Castán is a postdoctoral fellow in the Molecular Systems and nanoMaterials for Energy and Health (SyMMES) at the Atomic and Alternative Energies Commission (CEA) in Grenoble, France. He received his PhD in Materials Science in 2018 at the Université d’Angers in France. His research is focused on the synthesis of photochromic dyes for their use in Dye-Sensitized Solar Cells. He is the author of more than 20 indexed publications.
R. Demadrille is a team leader at the Atomic and Alternative Energies Commission (CEA) in France. He received his PhD in organic chemistry in 2000 from the University of Aix-Marseille with a grant from PPG Industries and Essilor International before to join the R&D department of an international chemical company to work on functional polymer materials. Then he moved to CEA as a postdoctoral fellow to develop semiconducting polymers for organic photovoltaics before being appointed in 2005 as a permanent researcher. His research focuses on the synthesis and the characterization of new pi-conjugated molecules and polymers for organic and hybrid photovoltaics and electronics. In 2018, he was recipient of the “Chemistry Energy” prize of the French Society of Chemistry, and in 2019, he was awarded of an ERC Advanced Grant to develop photochromic solar cells. Since 2020, he is Associate Editor of Journal of Materials Chemistry C and Materials Advances. He is the author of 8 patents, 2 book chapters and more than 90 articles indexed by SCI.