Cancer is a major health issue worldwide, and the development of innovative and effective drugs is an ultimate demand for research. Iron compounds have aroused a great interest in the search for new metal based chemotherapics, on account of their relatively low toxicity and their redox chemistry, exportable to physiological media.
Some ferrocene derivatives have shown a promising anticancer potential, with a strong activity mostly associated with FeII to FeIII oxidation, leading to alteration of the cellular redox balance and subsequent production of toxic substances (reactive oxygen species, ROS). However, they provide a limited variability of the metal coordination set, and they need to be carefully formulated for in vivo applications due to a generally insufficient water solubility.
Since 2019, a series of cationic [FeIFeI] complexes based on the [Fe2Cp2(CO)2] core and comprising a vinyliminium bridging ligand have emerged as a novel class of potential chemotherapeutic agents. Thanks to the unique features of the bimetallic core, these complexes are easily prepared up to gram scale from a commercial precursor in a few synthetic steps. Remarkably, they are amphiphilic and appreciably water-soluble, and exhibit an antiproliferative activity against cancer cell lines which depends on the ligand substituents. The choice of the latter is virtually limitless, thanks to the generality of the synthetic procedure, and this feature allows to optimise physico-chemical properties for biological purposes. Different mechanisms, mainly ROS production but also protein interaction and weak DNA binding, may contribute to the mode of action of these dinuclear structures.
Recently, the group of Fabio Marchetti and co-workers have reported, for the first time, the conjugation of a ferrocenyl moiety with a diiron framework, as a strategy to obtain robust mixed-valence triiron compounds featured by a potent cytotoxicity and excellent selectivity towards cancer cell lines (i.e., IC50 values in the low micromolar/nanomolar range on the cancer cell lines, and up to 35 times higher values on the nontumoral cells).
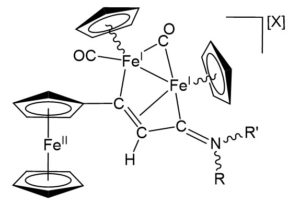
Figure 1. General structure of the novel triiron complexes derived from the tethering of a ferrocenyl unit and a di-organoiron core. R=Me, aryl, Bz, allyl; R’=Me, Bz; X=CF3SO3, NO3.
A combination of stability studies, electrochemical experiments, iron cellular uptake and targeted biological studies indicate that the cationic triiron complexes synergistically combine the redox behaviour of the ferrocenyl moiety with the amphiphilicity and the versatility of the diiron vinyliminium structure, and that their powerful activity arises from the ability to disrupt the redox homeostasis of tumour cells, through the overproduction of intracellular ROS and the alteration of the thioredoxin reductase, assessed on a synthetic dodecapeptide as a simplified model of the enzyme.
Corresponding Author:
Fabio Marchetti received his Degree in Industrial Chemistry from the University of Bologna in 1999 (summa cum laude), and the PhD in Chemistry from the same University in 2003. In 2006 he obtained a researcher position at the University of Pisa, and since October 2018 he has been Full Professor in the same University. FM has co-authored over 200 scientific publications on international journals, 2 book chapters and 2 international patents. His research interests regard the synthesis, the characterization and the properties of new transition metal compounds, and the metal-mediated activation of small organic molecules.