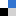Emerging Investigator: Hongwei Yu
 |
|
Read Hongwei Yu’s Emerging Investigator Series article on Inorganic Chemistry Frontiers and learn more about him.
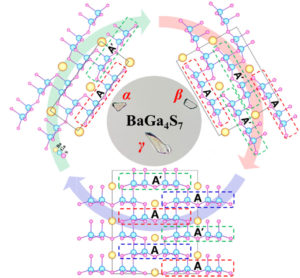
| The exploration of new infrared nonlinear optical crystals based on the polymorphism of BaGa4S7 | ||
| Zhen Qian, Haonan Liu, Yujie Zhang, Hongping Wu, Zhanggui Hu, Jiyang Wang, Yicheng Wu and Hongwei Yu* | ||
|
|
Two new polymorphism of BaGa4S7 was successfully discovered and synthesized. Among them, β-BaGa4S7 exhibits the best balance among a large phase-matching SHG response and a wide band gap, as well as the stable physicochemical property. |
|
 |
||
| From the themed collection: Frontiers Emerging Investigator Series | ||
| The article was first published on 26 Jul 2022 | ||
| Inorg. Chem. Front., 2022, Advance Article | ||
| https://doi.org/10.1039/D2QI01263D | ||
My research interests
| Key words: nonlinear optical crystals, solid state chemistry, crystal growth |
| Nonlinear optical (NLO) crystals—the unique materials capable of generating coherent radiation at various difficult-to-access wavelengths through frequency conversion technologies—are of particular importance for laser and photonic technologies. Currently, the commercial NLO crystals are mainly used in the ultraviolet (UV) and visible regions. However, in the deep-UV (λ < 200 nm) and mid-IR (3 μm < λ < 20 μm) regions, the available NLO crystals are still limited. Therefore, my research interests are to design, synthesize and grow new NLO crystals for the laser output in deep-UV and IR regions. The materials classes I am interested in include borates, phosphates, chalcogenides and some heteroanionic compounds, etc. |
10 Facts about me
| I published my first academic article on synthesis, structure and characterization of a new tripotassium cadmium pentaborate in Journal of Solid State Chemistry in 2011.
An accomplishment I’m particularly proud of is that I have synthesized hundreds of new inorganic crystals and determined their structures by single-crystal X-ray diffraction. My favourite sport is mountain-climbing. One of my hidden talents is singing. One thing I cannot live without is delicious food. My favorite books were tales of mystery when I was a child. I always believe that a good chemist would also be a good cooker. In five years, I hope to get an excellent NLO material for achieving highly effective output of deep-UV lasers. I chose chemistry as a career because chemistry is magical; it can create a new material world. The best advice I have ever been given is to cherish everything around you. |