With a limited amount of fossil fuels in the ground, developing alterative strategies for energy production is of the utmost importance. However, without a way to store that energy, these endeavors could be futile.
From the team of Gyeonghee Lee, Chakrapani V. Varansi, and Jie Liu (an Associate Editor here at Nanoscale), comes one of this month’s HOT papers, “Effects of morphology and chemical doping on electrochemical properties of metal hydroxides in pseudocapacitors.” In this paper, the team’s specific metal hydroxide of interest is Ni(OH)2.
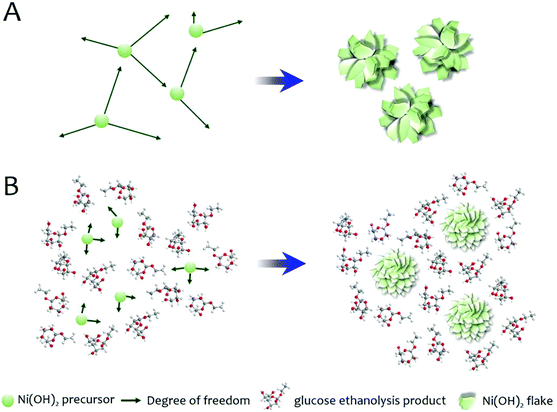
Schematic illustration of the proposed mechanism for the morphology modification of Ni(OH)2 by glucose in the solvothermal medium.
Lee et al. state “…that both morphology control and chemical doping positively affect the electrochemical performance of Ni(OH)2 when applied individually.” In this paper, they set out to determine what happens to the electrochemical performance when morphology and doping are combined instead of applied individually. In order to investigate the effects of both, they chose a solvothermal synthesis route for metal hydroxide flakes, creating both cobalt-doped (CoxNi1-x(OH)2) and undoped particles (Ni(OH)2) that have varying amounts of D-glucose added. For both the doped and undoped particles, four sets of particles are created, where 0, 10, 20, and 50% of the urea normally used in the solvothermal process is replaced by the D-glucose. All of the particles were characterized with x-ray diffraction (XRD) and scanning electron microscopy (SEM), with the SEM showing that increasing amounts of glucose decreased flake size. XRD and thermogravimetric analysis also revealed that increasing D-glucose amounts created an increase in the amount of interlayer water, with XRD also indicating degree of crystallinity decreased with increasing D-glucose.
In order to understand how the glucose affects morphology, the authors ran the solvothermal synthesis without the metal precursor. XRD and nuclear magnetic resonanace (NMR) data indicates the presence of ethyl-substituted glucose (formed during the synthesis process) instead of D-glucose. The authors conclude that “…ethyl glucoside molecules can hinder the precursor diffusion and thus inhibit the metal hydroxide growth during synthesis.”
The electrochemical performance of all of the types of particle is evaluated using cyclic voltammetry (CV), galvanostatic charge–discharge, and electrochemical impedance spectroscopy (EIS). Increasing glucose levels on undoped particles increased specific capacitance and cobalt doping is also shown to improve specific capacitance. Combining the two showed that specific capacitance increased up to 50% of Co doping, but was less effective above 50%.
Lee et al. find that “…the addition of glucose in the ethanol-mediated solvothermal synthesis effectively reduces the particle size of metal hydroxide flakes. The specific capacitance is improved as a result of the increased surface area and reduced particle size.” In terms of specific capacitance, the higher interlayer water levels found for increasing glucose levels may make ion mobility easier in the materials, enhancing the specific capacitance of the doped-Ni(OH)2. In the doped materials, the specific capacitance was increased with low doping and decreased with high doping.
In designing future iterations of Ni(OH)2 batteries, careful attention will need to be placed on both morphology and doping levels in order to find the best balance for electrochemical performance.
Effects of morphology and chemical doping on electrochemical properties of metal hydroxides in pseudocapacitors
Gyeonghee Lee, Chakrapani V. Varanasi and Jie Liu
Nanoscale, 2015, 7, 3181-3188. DOI: 10.1039/C4NR06997H
Stephanie E. Vasko is currently a Senior Research Assistant at The Rock Ethics Institute at the Pennsylvania State University in State College. Her research focuses on science communication, STEM education, and the intersections between art, craft, and science. You can follow her on Twitter at @stephanievasko.
The opinions and views expressed in this piece are those of the author and do not represent or reflect the opinion, views, or policy of the Pennsylvania State University, the Rock Ethics Institute, or the National Science Foundation.