Liquid crystal elastomers (LCEs) have emerged as a leading platform for soft actuators, haptic interfaces, and mechanically responsive materials, owing to their ability to undergo large, reversible shape transformations. Conventional two-stage LCE programming strategies, however, typically rely on a base-catalyzed first crosslinking step, which limits processing time, scalability, and compatibility with modern manufacturing techniques. At the same time, light-based 3D printing has transformed the fabrication of polymeric materials by offering precise spatiotemporal control through photopolymerization. To move beyond static structures and fully realize concepts such as 4D printing, where printed objects can change shape or function after fabrication, advanced chemistries are required that are fully compatible with these manufacturing workflows and enable post-fabrication shape programming.
In recent work published in Materials Horizons, Liu and co-workers introduce an elegant and powerful alternative: a two-stage wavelength-selective photopolymerization strategy that eliminates the need for base catalysis. Importantly, the fully light-driven nature of this approach opens new opportunities for advanced fabrication methodologies.
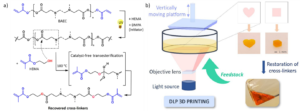
By employing a bifunctional acrylate–oxetane crosslinker together with two photoinitiators possessing non-overlapping absorption windows, the team independently trigger free-radical and cationic ring-opening polymerizations using different wavelengths of light (Figure 1a). This design provides unprecedented spatiotemporal control over the first crosslinking stage, enabling a stable, loosely crosslinked intermediate that can be processed in ways previously inaccessible to LCE systems.

Figure 1: (a) Schematic illustration of the two-step crosslinking strategy for LCEs and (b) light-based fabrication of a shape-memory actuator. Reproduced from DOI: 10.1039/D5MH01907A with permission from the Royal Society of Chemistry.
This enhanced processability allows LCE films to be coated, patterned, stretched, twisted, embossed, or directly ink-written prior to final network locking. The authors demonstrate a rich set of actuation modes, including uniaxial contraction, bending, twisting, dome-shaping, and dynamically reconfigurable surface topographies, with excellent fixity and repeatability. Particularly noteworthy is the ability to perform post-extrusion mechanical programming in direct-ink-written LCEs, introducing a new pathway to combine shear-induced alignment with deliberate mechanical deformation before final curing.
By replacing catalyst-triggered gelation with wavelength-orthogonal photoactivation, this work establishes a new manufacturing paradigm for LCE-based soft actuators. The approach significantly expands the accessible design space for shape-programmable materials, opening the door to advanced 4D printing, photopatterning, and mechanically complex shape programming (Figure 1b). As such, it represents an important step forward for the scalable fabrication of sophisticated soft, adaptive materials.
To find out more, please read:
Shape programming of liquid crystal elastomers by two-stage wavelength-selective photopolymerization
Tom Bruining, Daniela R. Tomé, Danqing Liu
Mater. Horiz., 2026, DOI: 10.1039/D5MH01907A
About the blogger
 |
Dr. Kostas Parkatzidis is a Swiss National Science Foundation Postdoc Fellow in the group of Professor Zhenan Bao at Stanford University (United States), working on the molecular design of polymer-based skin-inspired materials for various applications. Kostas obtained his PhD from ETH Zurich (Switzerland) under the supervision of Professor Athina Anastasaki where he focused on the development of advanced polymer synthesis and chemical recycling methodologies. He also holds MSc in Organic Chemistry and BSc in Materials Science and Technology obtained from the University of Crete (Greece). Since 2023, Kostas has served as a Materials Horizons Community Board member. |