3D printing has revolutionized the production of polymer-based materials, with the development of numerous printing techniques that continuously enhance speed, resolution, and accessibility. Among the most significant advancements is light-based 3D printing, which utilizes photopolymerization reactions to solidify inks, offering precise spatiotemporal control over the printing process. However, light-based 3D printing primarily relies on uncontrolled polymerization of (meth)acrylate and epoxide monomers/ cross-linkers, which yield non-functional, non-recyclable materials. To fully exploit the potential of 3D printing, there is growing interest in integrating advanced chemistries, such as dynamic chemistry, that enable the fabrication of functional materials capable of post-printing modifications in shape, properties, and functionality. Most polymer-based objects created through current 3D printing techniques cannot be chemically recycled due to the chemical inertness of the materials and the irreversible polymerization processes involved. Thus, it is imperative to incorporate new chemistries into 3D printing that enable the fabrication of advanced, functional, and chemically recyclable materials at the end of their lifecycle.
In a recent work by Du Prez and Nguyen, a closed-loop 3D printing process utilizing dynamic chemistry was reported. The authors synthesized an acrylate photocurable polymeric material based on dynamic β-amino ester cross-linkers, which can be used as an ink for 3D printing. Importantly, this material can undergo decross-linking via a transesterification reaction, resulting in the depolymerization of the formed polymer network. This process is not only reversible, enabling the making and unmaking of polymers, but it also operates under highly appealing and relatively mild conditions.
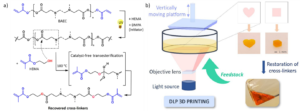
Figure 1: a) Chemical overview of the polymerization and depolymerization reactions of the dynamic network and b) closed-loop 3D printing. Image reproduced from DOI: 10.1039/D4MH00823E with permission from the Royal Society of Chemistry.
The team first synthesized an acrylate-terminated β-amino ester-containing cross-linker, which serves as a building block for the formation of a dynamic polymer network. This cross-linker was prepared via a one-step aza-Michael addition reaction. The process is straightforward and utilizes commercially available reagents, making the material easily adaptable for other researchers. These low-molecular-weight diacrylate polymers undergo photo-induced free radical polymerization, resulting in the formation of a dynamic polymer network.
This dynamic nature of the polymeric network enables thermomechanical reprocessing of the material. To demonstrate this aspect, the authors used compression molding (at 150 °C under 2 tons of pressure for 30 minutes) for reprocessability. The recycling procedure was repeated three times, with the material showing a slight increase in shear modulus and activation energy, while maintaining stable thermal properties and chemical integrity. The increase in temperature results in a reduction of cross-link density, demonstrating the role of dissociative exchange via (retro) aza-Michael addition. In contrast, polymeric networks without dynamic bonds did not exhibit any reprocessability, highlighting the promise of dynamic chemistry in enabling reprocessable materials.
In the next step, to demonstrate more advanced chemical recycling of this dynamic network, the team employed another reaction (transesterification) using a commercially available methacrylate with a hydroxyl moiety (hydroxyethyl methacrylate) as a decross-linker. The hydroxyl group of this functional methacrylate reacts with the β-amino ester, resulting in the decross-linking of the network and its depolymerization. This reaction is temperature-dependent and requires an optimized amount of decross-linker to achieve a high depolymerization yield. Notably, the depolymerization reaction proceeds without the need for any catalyst or solvent, making the procedure even more appealing. Upon depolymerization of the polymer network, the resulting mixture consists of methacrylate-terminated compounds, as confirmed by electrospray ionization mass spectrometry. These compounds can be further employed for another cycle of photo-curing, enabling the development of curable-depolymerizable dynamic materials.
In the final part, the team demonstrated the translation of this concept into closed-loop 3D printing using the dynamic photocurable resin. Multiple printing cycles were performed with recycled photocurable resin to assess the repeatability of this procedure. The resulting samples exhibited consistent shapes and appearances for at least three cycles, thus demonstrating repeatable printability after chemical recycling.
Overall, this study elegantly combines fundamental aspects of dynamic polymer chemistry and its application in 3D printing, further enhancing interest in dynamic chemistry for both functional and recyclable materials.
To find out more, please read:
Direct restoration of photocurable cross-linkers for repeated light-based 3D printing of covalent adaptable networks
Loc Tan Nguyen and Filip E. Du Prez
Mater. Horiz., 2024, DOI:10.1039/D4MH00823E
About the blogger

Dr. Kostas Parkatzidis is a Swiss National Science Foundation Postdoc Fellow in the group of Professor Zhenan Bao at Stanford University (United States), working on the molecular design of polymer-based skin-inspired materials for various applications. Kostas obtained his PhD from ETH Zurich (Switzerland) under the supervision of Professor Athina Anastasaki where he focused on the development of advanced polymer synthesis and chemical recycling methodologies. He also holds MSc in Organic Chemistry and BSc in Materials Science and Engineering obtained from the University of Crete (Greece). Since 2023, Kostas has served as a Materials Horizons Community Board member. |