Polymeric particles represent a widely utilized class of materials due to their adjustable size and shape, high volume-surface area ratio, customizable properties and ease of surface modification. These attributes make them indispensable across diverse fields, finding applications in coatings, pigments, drug delivery, nanomedicine, catalysis and more. Traditional methods of synthesis, predominantly via thermal heterogeneous polymerization like emulsion or dispersion polymerization, often necessitate the use of surfactants/stabilizers and thermal initiators, risking material contamination and complicating purification processes. In recent years, light polymerization has gained significant attention due to its ability to operate under milder conditions and offer temporal regulation. However, employing the photo-flow reaction, which is commonly viewed as an appealing method for upscaling reactions, proves challenging for heterogeneous systems due to constraints such as limited light penetration and scattering.
In a recent study by Holloway et al., an upscaled, photochemical synthesis of polymeric particles via flow chemistry was achieved based on precipitation step-growth polymerization without radical or surfactant sources. This innovative approach optimized an easy, scalable, and fast synthetic procedure and produced functional polymeric materials with chemiluminescent self-reporting properties and chemical-stimulated degradability, thus opening novel avenues for scaled-up synthesis of functional polymeric particles (Figure 1).
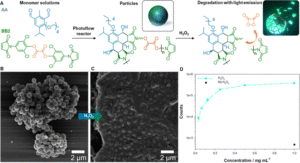
Figure 1: (A) Overview of the particle formation with AA and BB2 monomers and subsequent cleavage of the oxalate bond in presence of H2O2, resulting in the degradation of the particles and light emission. (B) SEM image of the particles after synthesis. (C) SEM image of the particles after adding H2O2. (D) Photocount recorded at various particles concentrations. Reproduced from DOI: 10.1039/D4MH00106K with permission from the Royal Society of Chemistry.
Harnessing their expertise in photochemical reactions, the team synthesized functional AA and BB monomers capable of Diels-Alder step-growth polymerization under light irradiation. The oligomers, with limited solubility in the reaction solvent, precipitated out upon reaching a critical molecular weight, facilitating particle formation via precipitation polymerization mechanism. Thorough optimization of polymerization parameters enabled precise control over particle size, yield, and shape, with solvent selection and flow rate playing crucial roles in the process. The solvent selection significantly affected polymer solubility, with dramatic effects on yield and particle size. The reaction yield increased impressively, from 1% in acetonitrile, a commonly used solvent, to up to 60% in the optimized water/methanol mixture. Since the reactions took place in a flow system, the flow rate was of paramount importance. In-depth investigation revealed that tuning the flow rate allowed manipulation of reaction yield, particle size, and shape, with the ability to tune the particle size over 545 nm (ranging from 185 to 730 nm). Furthermore, the initial monomer concentration played an important role in the reaction, as the limited solubility of the monomers prohibited equimolar monomer ratios at higher concentrations, significantly affecting particle morphology.
After successfully demonstrating the photo-flow reaction, the authors took advantage of the polymer’s nature, demonstrating its potential application in point-of-care devices. The Diels-Alder adduct exhibited intrinsic fluorescence, serving as a fluorophore for the peroxylate chemiluminescent reaction. Specifically, upon the addition of H2O2, the particles degraded via oxalate bond cleavage, resulting in the excitation of the fluorophore and subsequent photon emission with a strong signal, allowing for degradation monitoring.
In summary, this work surpasses previous approaches by developing a scalable photo-flow system capable of producing polymeric particles with tuneable properties within minutes, free from additives or radical initiators. The molecular design of these polymers enables the synthesis of functional and degradable particles responsive to chemical stimuli, featuring the potential for diverse applications. Overall, this study elegantly combines fundamental aspects of polymerization and materials design to pave the way for a plethora of applications in various fields.
To find out more, please read:
Photo-induced synthesis of polymeric nanoparticles and chemiluminescent degradable materials via flow chemistry
Joshua O. Holloway, Laura Delafresnaye, Emily M. Cameron, Jochen A. Kammerer and Christopher Barner-Kowollik
Mater. Horiz., 2024, DOI:10.1039/D4MH00106K
About the blogger

Dr. Kostas Parkatzidis is a Swiss National Science Foundation Postdoc Fellow in the group of Professor Zhenan Bao at Stanford University (United States), working on the molecular design of polymer-based skin-inspired materials for various applications. Kostas obtained his PhD from ETH Zurich (Switzerland) under the supervision of Professor Athina Anastasaki where he focused on the development of advanced polymer synthesis and chemical recycling methodologies. He also holds MSc in Organic Chemistry and BSc in Materials Science and Technology obtained from the University of Crete (Greece). Since 2023, Kostas has served as a Materials Horizons Community Board member. |