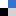Is it feasible to convert nitrogen to ammonia using water and light?
With international collaboration, scientists from China and Singapore have looked into the aspect of the state-of-the-art engineering of photocatalysts for the nitrogen (N2) fixation toward understanding the ammonia (NH3) synthesis. The work was recently reported by Dr. Wee-Jun Ong and co-workers in Materials Horizons, which is featured on the Inside Front Cover in Volume 5, Issue 1 in 2018.
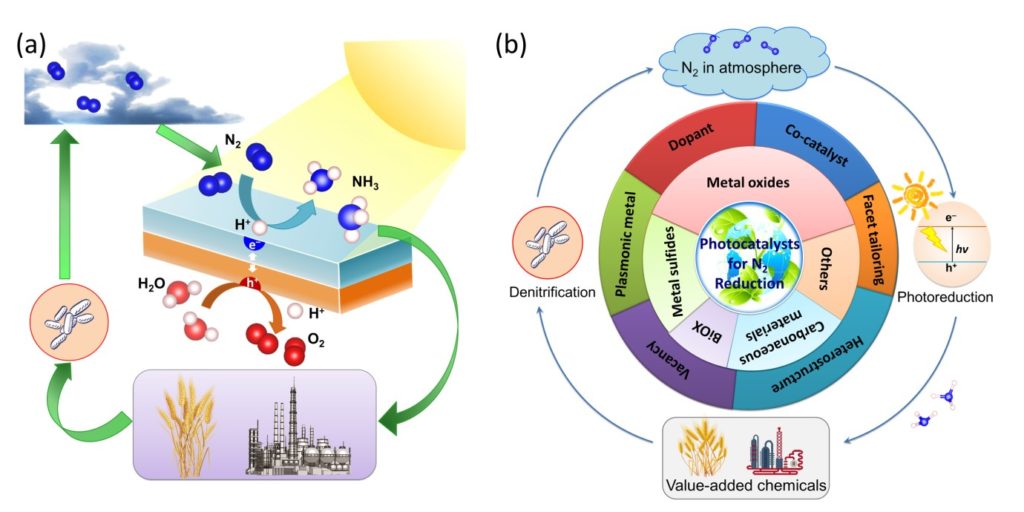
(a) An overview of the N2 cycle and circulation of N2 in various forms. (b) Diagram of the state-of-the-art milestone in the development of photocatalysts for N2 fixation. Images adapted from Chen et al., Mater. Horiz., 2018, Advance Article with permission from The Royal Society of Chemistry.
N2 is one of the most abundant gases on the Earth, comprising 78% in our atmosphere. Nonetheless, N2 in the gaseous state cannot be effectively utilized by organisms. Therefore, N2 must be “fixed” to make it valuable by breaking the strong NN triple bonds to transform it into a form that can be consumed by plants, animals and human beings. Hitherto, two typical methods to realize the fixation of N2 are: (1) a natural and bacterial process, and (2) the Haber-Bosch process in industry. For the last 100 years, the N2 conversion has led to the commercial fertilizer production and sustained the food intake supply for the worldwide population. However, the Haber-Bosch process consumes high pressures and temperatures, hence demanding a huge quantity (~2%) of the fossil fuel source. Thus, it is envisaged that the alternative process, which utilizes nanomaterials to absorb photon to mimic the natural photosynthesis in green leaves, can act as a paradigm shift for fixing nitrogen.
In this Review, the photo(electro)catalysts are classified based on the chemical compositions ranging from metal oxide to metal sulfide, bismuth oxyhalides, carbonaceous nanomaterials and other potential materials. The significance and relationship between the modification (e.g. nanoarchitecture design, crystal facet engineering, doping, and heterostructuring) and influences on the photo(electro)chemical activity of the catalysts are highlighted. Last but not least, to divert from the present laboratory-scale level to industrial applications, additional thoughts must be devoted to translating from academic research to practicality. How to amplify the yield of developed catalysts while preserving the intrinsic structures for the commercialization of “ammonia photosynthesis” is of universal challenge.
Wee-Jun Ong is a member of the Community Board for Materials Horizons. Currently, he works as a Staff Scientist in the Institute of Materials Research and Engineering (IMRE) at Agency for Science, Technology and Research (A*STAR) in Singapore. His research interests focus on photocatalytic, photoelectrochemical and electrochemical H2O splitting, CO2 reduction, N2 fixation and H2O2 production for energy conversion and storage via experimental and density functional theory (DFT) studies. At present, he also serves as the Associate Editor of Frontiers in Chemistry and Frontiers in Materials, and an Editorial Board Member of Scientific Reports, Nanotechnology and Nano Futures. Check out his personal research website here.