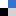Hybrid double perovskites have recently gained a vast amount of attention in the research area of photovoltaics as lead-free alternatives to the ground-breaking parent material [CH3NH3]PbI3.1 In double perovskites with the general formula A2B’B’’X6, two Pb2+ cations are effectively replaced with a monovalent B’ and a trivalent B’’ cation. Among the many fascinating properties of hybrid inorganic-organic perovskites, it is arguably the combination of strong light absorbance and long carrier lifetimes that make them so interesting for photovoltaic applications and light-emission devices. Recent experimental and theoretical studies on [CH3NH3]PbI3 revealed a direct-indirect character of the bandgap, i.e. [CH3NH3]PbI3 exhibits a direct band gap which is only approximately 47-60 meV higher in energy than the indirect band gap. Presumably, this is the origin of the paradox of strong absorption and long charge carrier lifetimes. When now turning our attention to lead free double perovskites, examples such as [CH3NH3]2KBiCl6 and [CH3NH3]2AgBiBr6 exhibit an indirect band gap,1 hence unfavourable light absorption properties. The symmetry mismatch that leads to the indirect band gap in such materials was recently studied by D. O. Scanlon and A. Zunger theoretically.2,3 Consequently, it is important to ask the question: is it possible to experimentally design a direct band gap in double perovskites?
In the recent article in Materials Horizons, ‘Designing Indirect-Direct Bandgap Transitions in Double Perovskites’,4 T. M. McQueen and co-workers have tackled this important question, studying the solid solution Cs2AgIn1-xSbxCl6 as a prototypical example. By wisely choosing B’ and B’’, a direct band gap in Cs2AgInCl6 has been achieved. The beauty lies in the simplicity of the concept – the understanding of band theory, i.e. symmetry and formation of bands with s-type and p-type character, see Figure 1. Going along the solid solution Cs2AgIn1-xSbxCl6, the valence band remains basically unchanged, whilst the character of the conduction band is continuously altered from s-type to p-type character. Clearly, the use of a chloride and in turn the ionic character of the solid with a band-gap larger than 3.5 eV limits the application of Cs2AgInCl6 in optoelectronics. However, the results depict a textbook example of how to manipulate properties in crystalline materials and open exciting opportunities for going forward in the field. For instance, one can easily envision a computational screening study of potential A2B’B’’X6 perovskites by using symmetry-based descriptors. Furthermore, it is important to note, that band engineering is a common concept in related areas of materials science, such as thermoelectrics and magnetic materials, and is a common tool for solid state chemists in general. Therefore, it is refreshing to see that band engineering now enters arguably one of the most fascinating developments of materials science within the last decade.
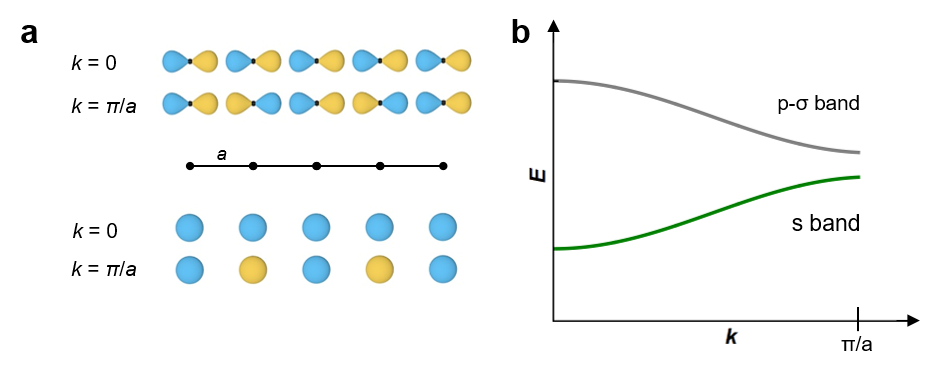
Figure 1. Schematic presentation of the orbital overlap (a) and the energy as a function of k for bands of s and p-σ orbitals (b) in a linear chain.
[1] F. Wei, Z. Deng, S. Sun, F. Xie, G. Kieslich, D. M. Evans, M. A. Carpenter, P. D. Bristowe, A. K. Cheetham ‘The synthesis, structure and electronic properties of a lead-free hybrid inorganic-organic double perovskites (MA)2KBiCl6 (MA = methylammonium)’ Mater. Horiz. 2016, 3, 328.
[2] C. N. Savory, A. Walsh and D. O. Scanlon ‘Can Pb-Free Halide Double Perovskites Support High-Efficiency Solar Cells?’ ACS Energy Lett. 2016, 1, 949.
[3] X.-G. Zhao, D. Yang, Y. Sun, T. Li, L. Zhang, L. Yu, A. Zunger ‘Cu-In Halide Perovskite Solar Absorbers’ J. Am. Chem. Soc. 2017, 139, 6718.
[4] T. Thao Tran, J. R. Panella, J. R. Chamorro, J. R. Morey, T. M. McQueen ‘Designing Indirect-Direct Bandgap Transitions in Double Perovskites’ Mater. Horiz. 2017, DOI: 10.1039/C7MH00239D.
Dr Gregor Kieslich is a Liebig-Fellow at Department of Chemistry, Technical University of Munich and is a member of the Community Board for Materials Horizons. He is an inorganic chemist focusing on crystal chemistry and structure–property relations in functional solids and hybrid frameworks: https://kieslichresearch.wordpress.com/