This month sees the following articles in MedChemComm that are in the top ten most accessed:
Towards biocompatible nanovalves based on mesoporous silica nanoparticles
Ying-Wei Yang
Med. Chem. Commun., 2011, 2, 1033-1049
DOI: 10.1039/C1MD00158B
Minisci reactions: Versatile CH-functionalizations for medicinal chemists
Matthew A. J. Duncton
Med. Chem. Commun., 2011, Advance Article
DOI: 10.1039/C1MD00134E
Are pyridazines privileged structures?
Camille G. Wermuth
Med. Chem. Commun., 2011, 2, 935-941
DOI: 10.1039/C1MD00074H
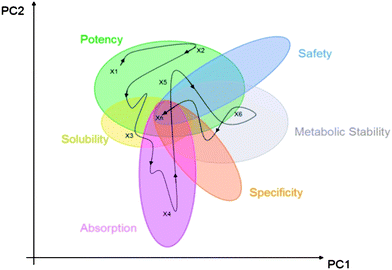
Molecular obesity, potency and other addictions in drug discovery
Michael M. Hann
Med. Chem. Commun., 2011, 2, 349-355
DOI: 10.1039/C1MD00017A
2-Anilinonicotinyl linked 1,3,4-oxadiazole derivatives: Synthesis, antitumour activity and inhibition of tubulin polymerization
Ahmed Kamal, Y. V. V. Srikanth, Thokhir B. Shaik, M. Naseer A. Khan, Md. Ashraf, M. Kashi Reddy, K. Anil Kumar and Shasi V. Kalivendi
Med. Chem. Commun., 2011, 2, 819-823
DOI: 10.1039/C0MD00177E
Potential atypical antipsychotics: synthesis, binding affinity and SAR of new heterocyclic bioisosteric butyrophenone analogues as multitarget ligands
María Barceló, Enrique Raviña, María J. Varela, José Brea, María I. Loza and Christian F. Masaguer
Med. Chem. Commun., 2011, Advance Article
DOI: 10.1039/C1MD00202C
Inhibitors of Stat5 protein signalling
Abbarna A. Cumaraswamy, Aleksandra Todic, Diana Resetca, Mark D. Minden and Patrick T. Gunning
Med. Chem. Commun., 2011, Advance Article
DOI: 10.1039/C1MD00175B
Structure–activity relationships of methyl-lysine reader antagonists
J. Martin Herold, Lindsey Ingerman James, Victoria K. Korboukh, Cen Gao, Kaitlyn E. Coil, Dennis J. Bua, Jacqueline L. Norris, Dmitri B. Kireev, Peter J. Brown, Jian Jin, William P. Janzen, Or Gozani and Stephen V. Frye
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C1MD00195G
Discovery of CP-866,087, a mu opioid receptor antagonist for the treatment of alcohol abuse and dependence
Stanton F. McHardy, Steven D. Heck, Sara Guediche, Monica Kalman, Martin P. Allen, Meihua Tu, Dianne K. Bryce, Anne W. Schmidt, Michelle Vanase-Frawley, Ernesto Callegari, Shawn Doran, Nicholas J. Grahame, Stafford McLean and Spiros Liras
Med. Chem. Commun., 2011, 2, 1001-1005
DOI: 10.1039/C1MD00164G
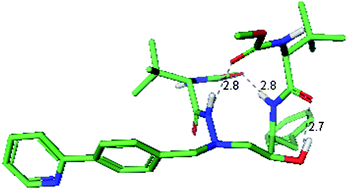
Intramolecular hydrogen bonding to improve membrane permeability and absorption in beyond rule of five chemical space
Alexander Alex, David S. Millan, Manuel Perez, Florian Wakenhut and Gavin A. Whitlock
Med. Chem. Commun., 2011, 2, 669-674
DOI: 10.1039/C1MD00093D
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to MedChemComm? Then why not submit to us today or alternatively email us your suggestions.
Comments Off on Top ten most accessed articles in October


![GA[7]](https://blogs.rsc.org/md/files/2011/12/GA7.gif)














![GA[3]](https://blogs.rsc.org/md/files/2011/11/GA31.gif)
