This month sees the following articles in MedChemComm that are in the top ten most accessed:
Oxadiazole isomers: all bioisosteres are not created equal
Kristin Goldberg, Sam Groombridge, Julian Hudson, Andrew G. Leach, Philip A. MacFaul, Adrian Pickup, Ruth Poultney, James S. Scott, Per H. Svensson and Joseph Sweeney
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C2MD20054F
Minisci reactions: Versatile CH-functionalizations for medicinal chemists
Matthew A. J. Duncton
Med. Chem. Commun., 2011, 2, 1135-1161
DOI: 10.1039/C1MD00134E
Gd(III) chelates for MRI contrast agents: from high relaxivity to “smart”, from blood pool to blood–brain barrier permeable
Chang-Tong Yang and Kai-Hsiang Chuang
Med. Chem. Commun., 2012, 3, 552-565
DOI: 10.1039/C2MD00279E
Small molecules DNA methyltransferases inhibitors
Nadine Martinet, Benoît Y. Michel, Philippe Bertrand and Rachid Benhida
Med. Chem. Commun., 2012, 3, 263-273
DOI: 10.1039/C1MD00194A
Determination of drug–receptor residence times by radioligand binding and functional assays: experimental strategies and physiological relevance
Georges Vauquelin
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C2MD20015E
Biosynthetic medicinal chemistry of natural product drugs
Frank E. Koehn
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C2MD00316C
Molecular obesity, potency and other addictions in drug discovery
Michael M. Hann
Med. Chem. Commun., 2011, 2, 349-355
DOI: 10.1039/C1MD00017A
Discovery of novel morpholino–quinoxalines as PI3Kα inhibitors by pharmacophore-based screening
Peng Wu, Yi Su, Xiaowen Liu, Jingying Yan, Yong Ye, Lei Zhang, Jianchao Xu, Shaoyu Weng, Yani Li, Tao Liu, Xiaowu Dong, Maotang Sun, Bo Yang, Qiaojun He and Yongzhou Hu
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C2MD00255H
A solid-phase method for peptide–siRNA covalent conjugates based on click chemistry
Yang Liu, Xiao-Feng Wang, Yue Chen, Li-He Zhang and Zhen-Jun Yang
Med. Chem. Commun., 2012, 3, 506-511
DOI: 10.1039/C2MD00198E
Targeting the inactive conformation of protein kinases: computational screening based on ligand conformation
Pascal Bonnet, Daniel Mucs and Richard A. Bryce
Med. Chem. Commun., 2012, 3, 434-440
DOI: 10.1039/C1MD00256B
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to MedChemComm? Then why not submit to us today or alternatively email us your suggestions.
Comments Off on Top ten most accessed article in April
 This striking cover brings the work of Patrick T. Gunning and co-workers to the forefront of issue 7.
This striking cover brings the work of Patrick T. Gunning and co-workers to the forefront of issue 7.










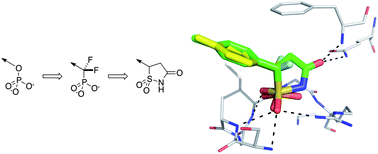 This review from
This review from 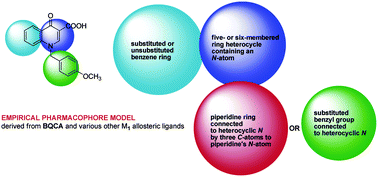
![GA[3]](https://blogs.rsc.org/md/files/2012/06/GA31.gif)
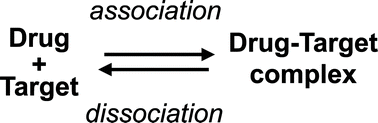 Previously drug–receptor interactions have only been quantified in terms of their affinity and efficacy but recently the residence time has also been recognized to affect the clinical performance.
Previously drug–receptor interactions have only been quantified in terms of their affinity and efficacy but recently the residence time has also been recognized to affect the clinical performance.