As an introduction to the web theme issue on Epigenetics published in MedChemComm (guest-edited by Dr Mark Bunnage – Pfizer – and Professor Rasmus Prætorius Clausen -University of Copenhagen), Nessa Carey (Head of Epigenetics ERDI, Pfizer) provides a concise summary of the current thinking in the ever expanding field of epigenetics.
Read Nessa’s review:
Epigenetics – an emerging and highly promising source of new drug targets
Nessa Carey
Med. Chem. Commun., 2012, Advance Article
DOI: 10.1039/C1MD00264C
…and view the collection of articles that makes for a timely and essential web theme issue in the emerging field of epigenetics research.


![GA[7]](https://blogs.rsc.org/md/files/2011/12/GA7.gif)









![GA[3]](https://blogs.rsc.org/md/files/2011/11/GA31.gif)
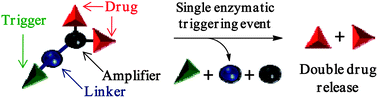
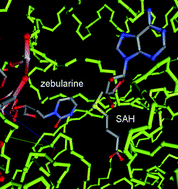
![GA[9]](https://blogs.rsc.org/md/files/2011/10/GA9-300x118.gif)
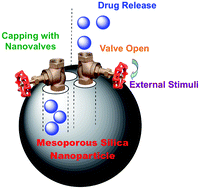
![CoverIssue[2]](https://blogs.rsc.org/md/files/2011/09/CoverIssue2.gif)