A challenge for the development of redox active metal-based chemotherapeutics is producing chemical nucleases capable of self-cleaving, so that they do not require a reducing agent to initiate DNA cleavage (which obviously limits their viability in vivo). In this HOT paper water soluble Cu2+ and Mn2+ bis-phenanthroline octanedioate complexes have been developed which are capable of doing just that – with very promising results.
A challenge for the development of redox active metal-based chemotherapeutics is producing chemical nucleases capable of self-cleaving, so that they do not require a reducing agent to initiate DNA cleavage (which obviously limits their viability in vivo). In this HOT paper water soluble Cu2+ and Mn2+ bis-phenanthroline octanedioate complexes have been developed which are capable of doing just that – with very promising results.
The self-cleaving chemical nucleases have been developed by Andrew Kellett and Michael Devereux from Dublin Institute of Technology and colleagues from the National University of Ireland, DuPont and Penn State University. The complexes show nano and picomolar in vitro cytotoxicity towards cancer cells and better drug tolerance in vivo than cisplatin.
To find out more, download the paper. This article is currently free to access and is on the cover of Issue 7.
Water-soluble bis(1,10-phenanthroline) octanedioate Cu2+ and Mn2+ complexes with unprecedented nano and picomolar in vitro cytotoxicity: promising leads for chemotherapeutic drug development
Andrew Kellett, Mark O’Connor, Malachy McCann, Orla Howe, Alan Casey, Pauraic McCarron, Kevin Kavanagh, Mary McNamara, Sean Kennedy, Donald D. May, Philip S. Skell, Denis O’Shea and Michael Devereux
Med. Chem. Commun., 2011, Advance Article
DOI: 10.1039/C0MD00266F
For the authors’ previous work why not also see:
Bis-phenanthroline copper(II) phthalate complexes are potent in vitro antitumour agents with ‘self-activating’ metallo-nuclease and DNA binding properties
Andrew Kellett, Mark O’Connor, Malachy McCann, Mary McNamara, Patrick Lynch, Georgina Rosair, Vickie McKee, Bernie Creaven, Maureen Walsh, Siobhan McClean, Agnieszka Foltyn, Denis O’Shea, Orla Howe and Michael Devereux
Dalton Trans., 2011, 40, 1024-1027
DOI: 10.1039/C0DT01607A











 The p53 signalling pathway is responsible for regulating the cell cycle, initiating DNA repair and triggering apoptosis where necessary. Its crucial importance can clearly be seen by the fact that about half of human cancers contain alterations in the p53 pathway. The proteins MDM2 and MDMX (a.k.a. MDM4) negatively regulate p53, and altered levels of these proteins are often deemed responsible for p53 alterations. As a result MDM2 has been extensively studied, but few MDM2 inhibitors have made it as far as clinical trials.
The p53 signalling pathway is responsible for regulating the cell cycle, initiating DNA repair and triggering apoptosis where necessary. Its crucial importance can clearly be seen by the fact that about half of human cancers contain alterations in the p53 pathway. The proteins MDM2 and MDMX (a.k.a. MDM4) negatively regulate p53, and altered levels of these proteins are often deemed responsible for p53 alterations. As a result MDM2 has been extensively studied, but few MDM2 inhibitors have made it as far as clinical trials. In this HOT paper David S. Millan and colleagues at Pfizer R&D explore the influence of intramolecular hydrogen bonding on the membrane permeability and bioavailability of some of these drugs. By determining the propensity of molecules to form intramolecular hydrogen bonds they are able to revise previous assumptions about where certain molecules sit in chemical space. They find that when hydrogen bonding is taken into account these drugs sit much closer to rule of five space – thereby explaining the successful absorption of drugs that otherwise violate rule of five principles.
In this HOT paper David S. Millan and colleagues at Pfizer R&D explore the influence of intramolecular hydrogen bonding on the membrane permeability and bioavailability of some of these drugs. By determining the propensity of molecules to form intramolecular hydrogen bonds they are able to revise previous assumptions about where certain molecules sit in chemical space. They find that when hydrogen bonding is taken into account these drugs sit much closer to rule of five space – thereby explaining the successful absorption of drugs that otherwise violate rule of five principles.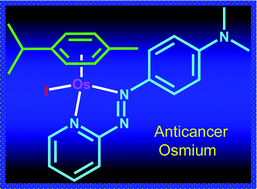 The majority of therapeutics clinically available for cancer treatment are platinum-based and work via a DNA alkylation mechanism that leads to cell apoptosis. But a number of cancers are resistant to apoptosis and not treatable with platinum-based drugs.
The majority of therapeutics clinically available for cancer treatment are platinum-based and work via a DNA alkylation mechanism that leads to cell apoptosis. But a number of cancers are resistant to apoptosis and not treatable with platinum-based drugs.