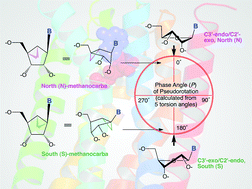
An approach to enhance the selectivity of these nucleoside and nucleotide derivatives is to make the ribose ring more rigid. This is achieved by using a methanocarba (bicyclo[3.1.0]hexane) ring system as a rigid substitution for ribose which maintains either a North (N) or South (S) conformation, preserving or enhancing the potency and/or selectivity for certain receptor subtypes.
This review from Dilip K. Tosh and Kenneth A. Jacobson of the National Institute of Diabetes and Digestive and Kidney Diseases summarises recent advances in the synthetic approaches to methanocarba derivatives in the context of their use as definitive pharmacological probes.
Methanocarba ring as a ribose modification in ligands of G protein-coupled purine and pyrimidine receptors: synthetic approaches
Dilip K. Tosh and Kenneth A. Jacobson
DOI: 10.1039/C2MD20348K










