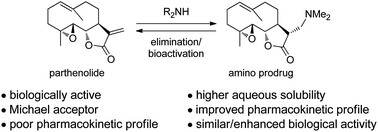
This review by David A. Colby et al. provides an interesting overview about an emerging prodrug approach that has been developed to overcome these problems in which an amine is added into the α-methylene-γ-lactone to mask this group from nucleophiles and increase solubility.
Included is a concise description of the medicinal chemistry of amino-derivatives of the sesquiterpene lactones, from early development in synthesis, to application in medicinal chemistry, to its move to a clinical candidate.
For the complete picture on this prodrug approach download the review now by following the link below…
Amino-derivatives of the sesquiterpene lactone class of natural products as prodrugs
James R. Woods, Huaping Mo, Andrew A. Bieberich, Tanja Alavanja and David A. Colby
DOI: 10.1039/C2MD20172K
| The review features as part of MedChemComm’s New Talent issue; view the expanding collection of articles for this issue HERE |