 Natural product isolation has developed as techniques have advanced e.g. improvements in chromatographic methodologies, however isolating labile natural products remains a large difficulty due to their inherent instability. Such is their instability that even the removal of solvents in vacuo, a common step during isolation, can destroy them. As such new careful isolation techniques are needed to offer access to new natural products and their biosynthetic intermediates.
Natural product isolation has developed as techniques have advanced e.g. improvements in chromatographic methodologies, however isolating labile natural products remains a large difficulty due to their inherent instability. Such is their instability that even the removal of solvents in vacuo, a common step during isolation, can destroy them. As such new careful isolation techniques are needed to offer access to new natural products and their biosynthetic intermediates.
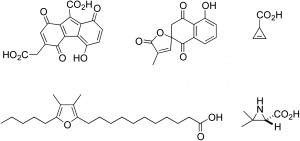
In this review Toshiyuki Wakimoto and Ikuro Abe discuss the current success in isolating labile natural products. Examples highlighted by Wakimoto and Abe include:
–Red pigments from a hippopotamus
-Labile polyketides from filamentous fungi
-Cyclopropene carboxylic acids from mushrooms
-Aziridine carboxylic acids from mushrooms
-Furan fatty acids from mussels
Labile natural products
Toshiyuki Wakimoto and Ikuro Abe
DOI: 10.1039/C2MD20016C
This review is part of MedChemComm’s Natural Products themed issue. Check out the rest of the articles appearing in this issue today…..










