Cancer tumors are a lot more complex than we think: besides cancer cells, supportive tissue cells, fat, and even immune cells can be found in a tumor. Combined crosstalk in between these cell groups influences the way the tumor develops or responses to drug treatment. On the other hand, the majority of what we know about cancer tumors has been acquired by studying cell ensembles. Recent strides to improve our understanding of cancer revealed that we have long been missing the stochastic interactions and rare events due to ensemble-average measurements. We can unveil how these cell groups work together and how the rare events change the fate of a tumor thanks to single-cell analysis techniques.
Single cells can be identified by extrinsic and intrinsic markers. Extrinsic markers are definitive of genetic and proteomic states of a cell. Flow cytometry and mass spectrometry have been the workhorse of extrinsic marker analysis, where genetic or proteomic materials are often fluorescently labeled for detection. With these techniques, multiplexed analysis of thousands of cells can be employed simultaneously. Intrinsic markers include size, shape, density, optical, mechanical, and electrical properties which do not require labelling. Microfluidic techniques provide with a plethora of different functionalities to sort the cells based on intrinsic markers. Combination of both extrinsic and intrinsic data advances our understanding of how cell heterogeneity is reflected in cell-to-cell variations in tumor development and drug-response. Although many powerful methods are available for determining extrinsic markers, not many techniques can gather information about a panel of different intrinsic markers.
A recent study from Biological Microtechnology and BioMEMS group at MIT represents an important microfluidic approach for the development of multiparameter intrinsic cytometry tool. The approach includes several different microfluidic modules combined with microscope imaging and image processing by machine learning. Separate modules measuring cell size, deformability, and polarization can be combined and organized within the tool (Figure 1). (i) Size module detects the cell size optically in a flow through system. Cell size module is necessary to separate different cell types that can give important cues about disease state. (ii) In deformability module, cells pass through narrow channels, and their transit time defines the deformability. Cell deformability gives cues about cytoskeletal and nuclear changes associated with cancer progression. (iii) In the polarization module, dielectrophoretic force at a fixed frequency is applied on cells driven by opposing hydrodynamic forces. Cells approach coplanar electrodes with different equilibrium positions depending on their polarizability. Cell polarizability allows for distinguishing subtle changes in biological phenotypes. As a proof-of-concept work, drug-induced structural changes in cells were detected for the first time using five different intrinsic markers, including size, deformability, and polarizability at three frequencies. The authors indicate that this powerful tool can further be equipped with visual readout capabilities, such as deterministic lateral displacement array, inertial microfluidics, acoustophoresis, optical techniques.
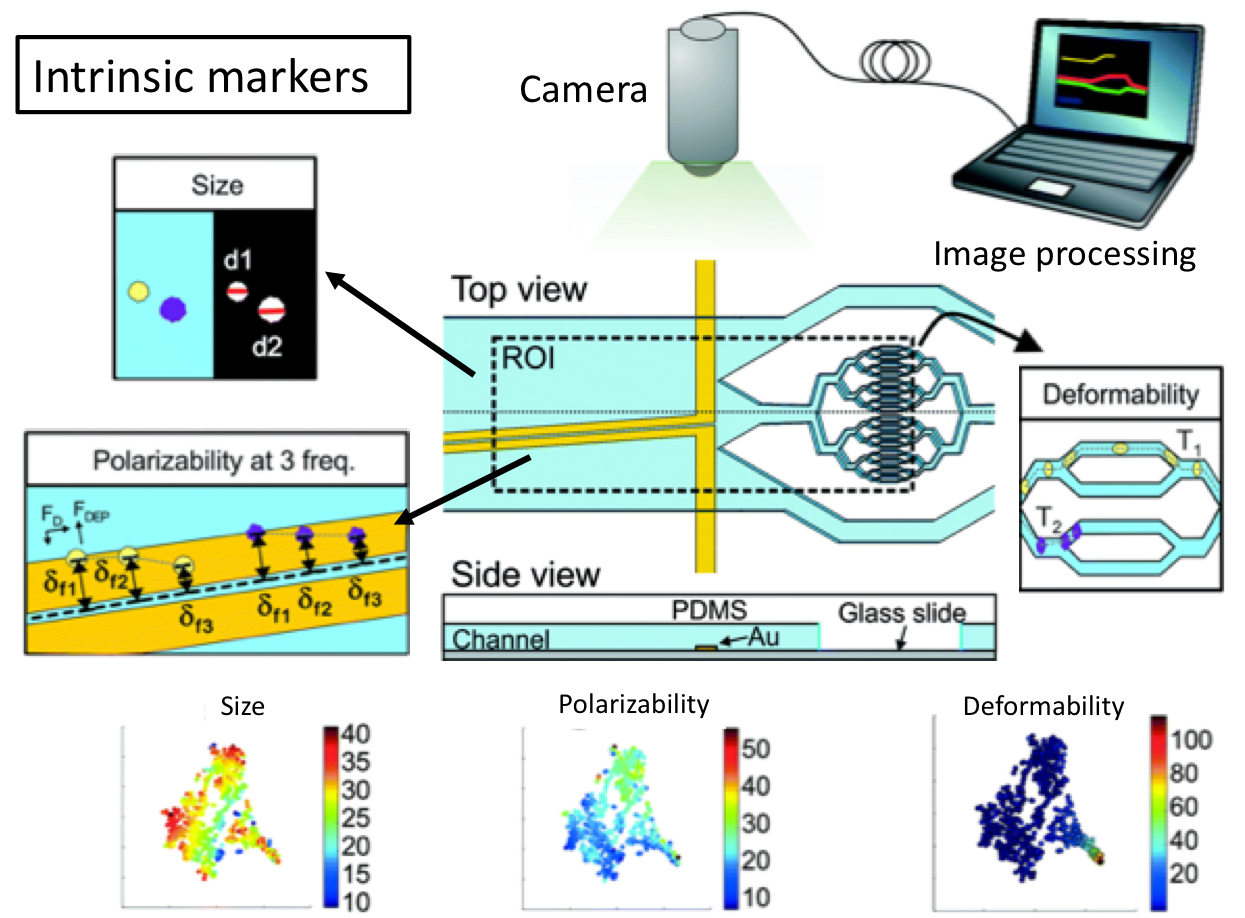
Figure 1. Multiparameter intrinsic cytometry combines different microfluidic modules on one substrate along with cell tracking to correlate per-cell information across modules for different intrinsic properties including size, polarizability, and deformability.
To download the full article for free* click the link below:
Multiparameter cell-tracking intrinsic cytometry for single-cell characterization
Apichitsopa, A. Jaffe, and J. Voldman
Lab Chip, 2018, Lab on a Chip Recent Hot Articles
DOI: 10.1039/C8LC00240A
*Article free to read until 31st August 2018
About the Webwriter

Burcu Gumuscu is a postdoctoral fellow in Herr Lab at UC Berkeley in the United States. Her research interests include development of microfluidic devices for quantitative analysis of proteins from single-cells, next generation sequencing,compartmentalized organ-on-chip studies, and desalination of water on the microscale.