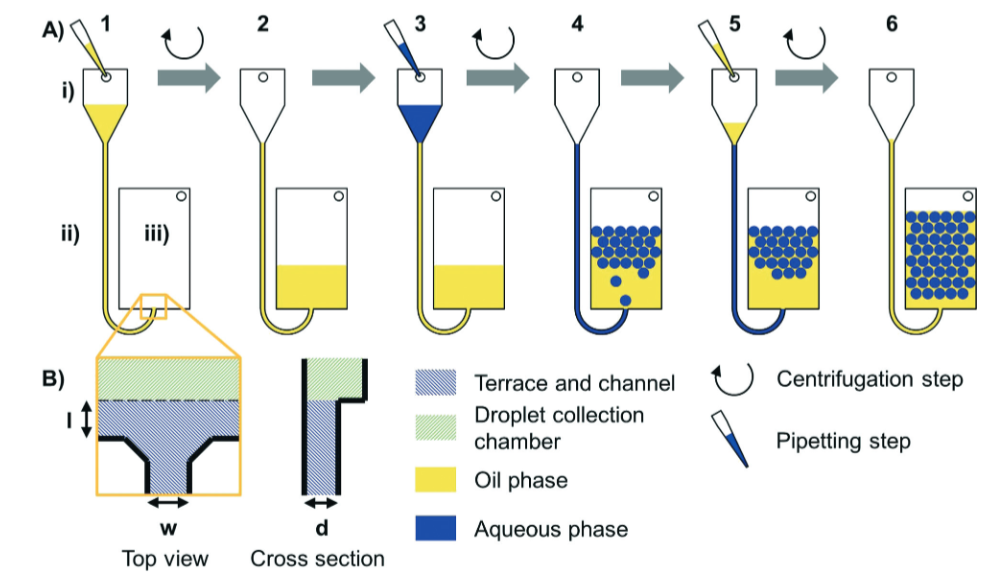
A group of scientists at IMTEK, University of Freiburg have developed a new method for the production of monodisperse droplets. Previous methods, such as T-junctions and flow focusing require several channels, containing either the disperse phase (which will form the droplet) or the continuous phase (which will surround the droplet), with the droplets forming at a constriction point in the tubing. Extremely precise control of flow rate is therefore required in order to achieve consistent droplet diameters. These methods also have substantial dead volumes due to sample material remaining in the tubing at the end of the process.
An alternative method is step emulsification (as highlighted recently in a Lab on a Chip HOT article). This only requires one channel, containing both phases, and the droplet formation is caused by a change in capillary pressure. The droplet size depends on the nozzle, rather than on pressure and flow rate, so this method is less sensitive to fluctuations than the methods mentioned previously. The main limitation of step emulsification is the relatively low throughput due to droplet accumulation at the nozzle. This publication reports the use of centrifugal force in order to solve this problem.
By spinning the whole system, the disperse phase (water) is overpressured relative to the continuous phase (oil), resulting in the droplet being forced away from the nozzle by the centrifugal gravitational field (step 3 to 4 above). In order to avoid sample material being wasted as dead volume, an additional aliquot of oil is added in order to push the last few droplets of water out of the nozzle (step 5 to 6 above).
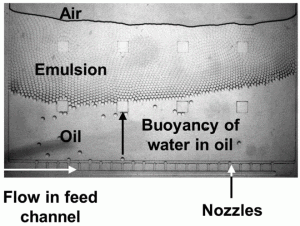
The authors found that droplet diameter is controlled by the nozzle geometry, while rate of formation is controlled by spinning frequency. In light of these findings, they were able to increase droplet production rate from less than 1 droplet per second, to greater than 500 droplets per second, while maintaining monodispersity. They were also able to set up multiple nozzles in parallel (as seen in the microscopic image), all feed by a larger channel, to further increase throughput.
In order to demonstrate the potential applications of this new method, the authors performed digital droplet recombinase polymerase amplification (ddRPA) of L. monocytogenes (a potential contaminant during food production). They found that the number of copies measured with ddRPA was consistent with those measured with digital droplet PCR, and the overall processing was 30 minutes, compared with 2 hourlis for ddPCR.
ddRPA is just one small example of how this new technique can be used – there are a huge range of potential applications where formation of monodisperse particles are a requirement and hopefully we will see this new method being adopted!
To download the full article for free click the link below:
Centrifugal step emulsification applied for absolute quantification of nucleic acids by digital droplet RPA
Friedrich Schuler, Frank Schwemmer, Martin Trotter, Simon Wadle, Roland Zengerle, Felix von Stetten and Nils Paust
DOI: 10.1039/C5LC00291E