This month sees the following articles in Lab on a Chip that are in the top ten most accessed:
A low cost point-of-care viscous sample preparation device for molecular diagnosis in the developing world; an example of microfluidic origami
A. V. Govindarajan, S. Ramachandran, G. D. Vigil, P. Yager and K. F. Böhringer
Lab Chip, 2012, 12, 174-181
DOI: 10.1039/C1LC20622B
A new method of UV-patternable hydrophobization of micro- and nanofluidic networks
Rerngchai Arayanarakool, Lingling Shui, Albert van den Berg and Jan C. T. Eijkel
Lab Chip, 2011, 11, 4260-4266
DOI: 10.1039/C1LC20716D
Highly-integrated lab-on-chip system for point-of-care multiparameter analysis
Soeren Schumacher, Jörg Nestler, Thomas Otto, Michael Wegener, Eva Ehrentreich-Förster, Dirk Michel, Kai Wunderlich, Silke Palzer, Kai Sohn, Achim Weber, Matthias Burgard, Andrzej Grzesiak, Andreas Teichert, Albrecht Brandenburg, Birgit Koger, Jörg Albers, Eric Nebling and Frank F. Bier
Lab Chip, 2012, 12, 464-473
DOI: 10.1039/C1LC20693A
Surfactants in droplet-based microfluidics
Jean-Christophe Baret
Lab Chip, 2012, 12, 422-433
DOI: 10.1039/C1LC20582J
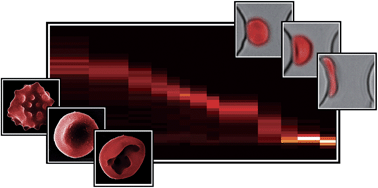
Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices
Xiaoxi Yang, Omid Forouzan, Theodore P. Brown and Sergey S. Shevkoplyas
Lab Chip, 2012, 12, 274-280
DOI: 10.1039/C1LC20803A
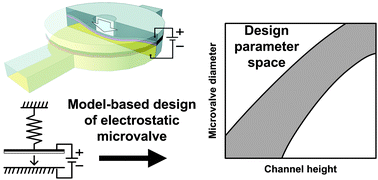
Photolithographic surface micromachining of polydimethylsiloxane (PDMS)
Weiqiang Chen, Raymond H. W. Lam and Jianping Fu
Lab Chip, 2012, 12, 391-395
DOI: 10.1039/C1LC20721K
A digital microfluidic platform for primary cell culture and analysis
Suthan Srigunapalan, Irwin A. Eydelnant, Craig A. Simmons and Aaron R. Wheeler
Lab Chip, 2012, 12, 369-375
DOI: 10.1039/C1LC20844F
Simultaneous high speed optical and impedance analysis of single particles with a microfluidic cytometer
David Barat, Daniel Spencer, Giuseppe Benazzi, Matthew Charles Mowlem and Hywel Morgan
Lab Chip, 2012, 12, 118-126
DOI: 10.1039/C1LC20785G
Ensembles of engineered cardiac tissues for physiological and pharmacological study: Heart on a chip
Anna Grosberg, Patrick W. Alford, Megan L. McCain and Kevin Kit Parker
Lab Chip, 2011, 11, 4165-4173
DOI: 10.1039/C1LC20557A
Fuel cell-powered microfluidic platform for lab-on-a-chip applications
Juan Pablo Esquivel, Marc Castellarnau, Tobias Senn, Bernd Löchel, Josep Samitier and Neus Sabaté
Lab Chip, 2012, 12, 74-79
DOI: 10.1039/C1LC20426B
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.













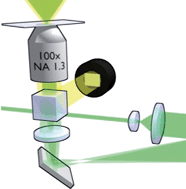 Holographic optical tweezers and their relevance to lab on chip devices
Holographic optical tweezers and their relevance to lab on chip devices  Optoelectrofluidic platforms for chemistry and biology
Optoelectrofluidic platforms for chemistry and biology
 The recent LOC article from Douglas Weibel and team at the University of Wisconsin-Madison has been highlighted on MedGadget. The article describes a new portable self-loading technology for determining minimum inhibitory concentration values, vital in clinical bacteriology for determining whether an organism is reported susceptible or resistant.
The recent LOC article from Douglas Weibel and team at the University of Wisconsin-Madison has been highlighted on MedGadget. The article describes a new portable self-loading technology for determining minimum inhibitory concentration values, vital in clinical bacteriology for determining whether an organism is reported susceptible or resistant.