View the new videos on the Lab on a Chip YouTube site using the links below:
Single exposure fabrication and manipulation of 3D hydrogel cell microcarriers
View the new videos on the Lab on a Chip YouTube site using the links below:
Single exposure fabrication and manipulation of 3D hydrogel cell microcarriers
This month sees the following articles in Lab on a Chip that are in the top ten most accessed:-
Cell lysis and DNA extraction of gram-positive and gram-negative bacteria from whole blood in a disposable microfluidic chip
Madhumita Mahalanabis, Hussam Al-Muayad, M. Dominika Kulinski, Dave Altman and Catherine M. Klapperich
Lab Chip, 2009, 9, 2811-2817, DOI: 10.1039/B905065P , Paper
Programmable diagnostic devices made from paper and tape
Andres W. Martinez, Scott T. Phillips, Zhihong Nie, Chao-Min Cheng, Emanuel Carrilho, Benjamin J. Wiley and George M. Whitesides
Lab Chip, 2010, 10, 2499-2504, DOI: 10.1039/C0LC00021C , Paper
Sickling of red blood cells through rapid oxygen exchange in microfluidic drops
Paul Abbyad, Pierre-Louis Tharaux, Jean-Louis Martin, Charles N. Baroud and Antigoni Alexandrou
Lab Chip, 2010, 10, 2505-2512, DOI: 10.1039/C004390G , Paper
Microstructuring of polymer films for sensitive genotyping by real-time PCR on a centrifugal microfluidic platform
Maximilian Focke, Fabian Stumpf, Bernd Faltin, Patrick Reith, Dylan Bamarni, Simon Wadle, Claas Müller, Holger Reinecke, Jacques Schrenzel, Patrice Francois, Daniel Mark, Günter Roth, Roland Zengerle and Felix von Stetten
Lab Chip, 2010, 10, 2519-2526, DOI: 10.1039/C004954A , Paper
Precompetitive preclinical ADME/Tox data: set it free on the web to facilitate computational model building and assist drug development
Sean Ekins and Antony J. Williams
Lab Chip, 2010, 10, 13-22, DOI: 10.1039/B917760B , Perspective
A microfluidic platform for probing small artery structure and function
Axel Günther, Sanjesh Yasotharan, Andrei Vagaon, Conrad Lochovsky, Sascha Pinto, Jingli Yang, Calvin Lau, Julia Voigtlaender-Bolz and Steffen-Sebastian Bolz
Lab Chip, 2010, 10, 2341-2349, DOI: 10.1039/C004675B , Paper
Predictive model for the size of bubbles and droplets created in microfluidic T-junctions
Volkert van Steijn, Chris R. Kleijn and Michiel T. Kreutzer
Lab Chip, 2010, 10, 2513-2518, DOI: 10.1039/C002625E , Paper
Agarose droplet microfluidics for highly parallel and efficient single molecule emulsion PCR
Xuefei Leng, Wenhua Zhang, Chunming Wang, Liang Cui and Chaoyong James Yang
Lab Chip, 2010, 10, 2841-2843, DOI: 10.1039/C0LC00145G , Communication
Research Highlights
Petra S. Dittrich
Lab Chip, 2010, 10, 2495-2496, DOI: 10.1039/C0LC90045A , Highlight
Electrochemical sensing in paper-based microfluidic devices
Zhihong Nie, Christian A. Nijhuis, Jinlong Gong, Xin Chen, Alexander Kumachev, Andres W. Martinez, Max Narovlyansky and George M. Whitesides
Lab Chip, 2010, 10, 477-483, DOI: 10.1039/B917150A , Paper
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.
View the new videos on the Lab on a Chip YouTube site using the links below:
Enhancement by optical force of separation in pinched flow fractionation
View the new videos on the Lab on a Chip YouTube site using the links below:
Sub-pixel resolving optofluidic microscope for on-chip cell imaging
A microdroplet-based shift register
A stretchable radio frequency (RF) radiation sensor that combines a microfluidic antenna and rigid electronic circuits has been developed by scientists in Sweden. This could open the way to reliable and durable second skin sensors for monitoring health.
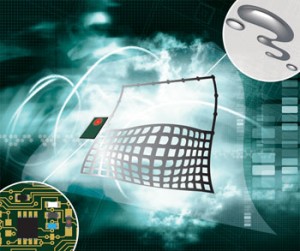 Flexible electronics are used in applications such as cameras, computer keyboards and photovoltaic cells. Some success has been found with stretchable antennas but the connection between the stretchable material and the rigid circuits still results in strain and loss of device sensitivity. To make wearable devices, electronics not only need to be flexible but they also need to be stretchable to truly conform to skin. Unfortunately, development from a flexible to a stretchable device has remained an elusive goal.
Flexible electronics are used in applications such as cameras, computer keyboards and photovoltaic cells. Some success has been found with stretchable antennas but the connection between the stretchable material and the rigid circuits still results in strain and loss of device sensitivity. To make wearable devices, electronics not only need to be flexible but they also need to be stretchable to truly conform to skin. Unfortunately, development from a flexible to a stretchable device has remained an elusive goal.
Now, Shi Cheng and Zhigang Wu from Uppsala University have developed a hybrid technology that combines conventional rigid circuitry with a substrate making a device that can bend, twist and stretch
IMAGE: Flexible microfluidic sensor responds to radio frequency signals
Click here to read the full story
Link to journal article
Microfluidic stretchable RF electronics
Shi Cheng and Zhigang Wu, Lab Chip, 2010
DOI: 10.1039/c005159d
This month sees the following articles in Lab on a Chip that are in the top ten most accessed:-
Cell lysis and DNA extraction of gram-positive and gram-negative bacteria from whole blood in a disposable microfluidic chip
Madhumita Mahalanabis, Hussam Al-Muayad, M. Dominika Kulinski, Dave Altman and Catherine M. Klapperich
Lab Chip, 2009, 9, 2811 – 2817, DOI: 10.1039/b905065p
Programmable diagnostic devices made from paper and tape
Andres W. Martinez, Scott T. Phillips, Zhihong Nie, Chao-Min Cheng, Emanuel Carrilho, Benjamin J. Wiley and George M. Whitesides
Lab Chip, 2010, 10, 2499 – 2504, DOI: 10.1039/c0lc00021c
Microfluidics without pumps: reinventing the T-sensor and H-filter in paper networks
Jennifer L. Osborn, Barry Lutz, Elain Fu, Peter Kauffman, Dean Y. Stevens and Paul Yager
Lab Chip, 2010, DOI: 10.1039/c004821f
Lab-on-a-chip devices as an emerging platform for stem cell biology
Kshitiz Gupta, Deok-Ho Kim, David Ellison, Christopher Smith, Arnab Kundu, Jessica Tuan, Kahp-Yang Suh and Andre Levchenko
Lab Chip, 2010, 10, 2019 – 2031, DOI: 10.1039/c004689b, Tutorial Review
Massively parallel detection of gene expression in single cells using subnanolitre wells
Yuan Gong, Adebola O. Ogunniyi and J. Christopher Love
Lab Chip, 2010, 10, 2334 – 2337, DOI: 10.1039/c004847j, Communication
Dynamics of microfluidic droplets
Charles N. Baroud, Francois Gallaire and Rémi Dangla
Lab Chip, 2010, 10, 2032 – 2045, DOI: 10.1039/c001191f, Critical Review
Precompetitive preclinical ADME/Tox data: set it free on the web to facilitate computational model building and assist drug development
Sean Ekins and Antony J. Williams
Lab Chip, 2010, 10, 13 – 22, DOI: 10.1039/b917760b, Perspective
Electrochemical sensing in paper-based microfluidic devices
Zhihong Nie, Christian A. Nijhuis, Jinlong Gong, Xin Chen, Alexander Kumachev, Andres W. Martinez, Max Narovlyansky and George M. Whitesides
Lab Chip, 2010, 10, 477 – 483, DOI: 10.1039/b917150a
Vortex-assisted DNA delivery
Jun Wang, Yihong Zhan, Victor M. Ugaz and Chang Lu
Lab Chip, 2010, 10, 2057 – 2061, DOI: 10.1039/c004472e
Shrink film patterning by craft cutter: complete plastic chips with high resolution/high-aspect ratio channel
Douglas Taylor, David Dyer, Valerie Lew and Michelle Khine
Lab Chip, 2010, 10, 2472 – 2475, DOI: 10.1039/c004737f, Technical Note
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.
Optoelectronic tweezers are able to distinguish between live and dead sperm cells, even if they aren’t moving, say US scientists.
To combat this problem, Aaron Ohta at the University of Hawaii and his team have demonstrated that optoelectronic tweezers – which use a combination of light and electric fields to control microscopic objects – can distinguish and sort between live and dead cells, irrespective of mobility.
Link to journal article
Motile and non-motile sperm diagnostic manipulation using optoelectronic tweezers
Aaron T. Ohta, Maurice Garcia, Justin K. Valley, Lia Banie, Hsan-Yin Hsu, Arash Jamshidi, Steven L. Neale, Tom Lue and Ming C. Wu, Lab Chip, 2010, DOI: 10.1039/c0lc00072h
Scientists in Australia have built a microneedle device capable of detecting disease-specific proteins directly from the skin.
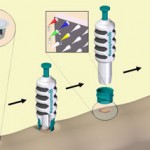 Normally when a clinical sample such as blood is needed to screen a patient for disease, it has to be taken by a specially trained healthcare practitioner using a needle and syringe. The sample is then clotted, centrifuged and stored under controlled conditions ready for analysis.
Normally when a clinical sample such as blood is needed to screen a patient for disease, it has to be taken by a specially trained healthcare practitioner using a needle and syringe. The sample is then clotted, centrifuged and stored under controlled conditions ready for analysis.
Now Mark Kendall and his colleagues from the University of Queensland, Australia have found an alternative pain-free method which dispenses with invasive needles, specialist training and sample processing. Kendall incorporated a small chip coated with sharp, densely packed microneedles into a patch that can be applied to the skin. The sharp gold-coated silicon needles are less than 1 mm in length and are able to capture and sample protein antibodies directly from the skin.
Link to journal article
Surface-modified microprojection arrays for intradermal biomarker capture, with low non-specific protein binding
Simon R. Corrie, Germain J. P. Fernando, Michael L. Crichton, Marion E. G. Brunck, Chris D. Anderson and Mark A. F. Kendall, Lab Chip, 2010
DOI: 10.1039/c0lc00068j
Issue 18 of Lab on a Chip is a special themed issue dedicated to Emerging Investigators in microfluidics guest-edited by Aaron Wheeler and Amy Herr.
The range of topics covered in this special issue of Lab on a Chip highlights the breadth of challenges currently being tackled by emerging investigators in the field. In the words of Guest Editor Amy Herr: “This issue paints a picture of the problems that the new generation of scientists in our field identifies as being important—and the broad impact their contributions are making across a wide range of disciplines”. Take a look now at this issue.
Here are a few highlights from the issue:
This month sees the following articles in Lab on a Chip that are in the top ten most accessed:-
Lensfree microscopy on a cellphone
Derek Tseng, Onur Mudanyali, Cetin Oztoprak, Serhan O. Isikman, Ikbal Sencan, Oguzhan Yaglidere and Aydogan Ozcan
Lab Chip, 2010, 10, 1787-1792, DOI: 10.1039/C003477K
Vortex-assisted DNA delivery
Jun Wang, Yihong Zhan, Victor M. Ugaz and Chang Lu
Lab Chip, 2010, 10, 2057-2061, DOI: 10.1039/C004472E
Centrifugal microfluidics for biomedical applications
Robert Gorkin, Jiwoon Park, Jonathan Siegrist, Mary Amasia, Beom Seok Lee, Jong-Myeon Park, Jintae Kim, Hanshin Kim, Marc Madou and Yoon-Kyoung Cho
Lab Chip, 2010, 10, 1758-1773, DOI: 10.1039/B924109D
Cell lysis and DNA extraction of gram-positive and gram-negative bacteria from whole blood in a disposable microfluidic chip
Madhumita Mahalanabis, Hussam Al-Muayad, M. Dominika Kulinski, Dave Altman and Catherine M. Klapperich
Lab Chip, 2009, 9, 2811-2817, DOI: 10.1039/B905065P
Digital PCR on a SlipChip
Feng Shen, Wenbin Du, Jason E. Kreutz, Alice Fok and Rustem F. Ismagilov
Lab Chip, 2010, Advance Article, DOI: 10.1039/C004521G
Hydrophilic PDMS microchannels for high-throughput formation of oil-in-water microdroplets and water-in-oil-in-water double emulsions
Wolfgang-Andreas C. Bauer, Martin Fischlechner, Chris Abell and Wilhelm T. S. Huck
Lab Chip, 2010, 10, 1814-1819, DOI: 10.1039/C004046K
A microfluidic platform for probing small artery structure and function
Axel Günther, Sanjesh Yasotharan, Andrei Vagaon, Conrad Lochovsky, Sascha Pinto, Jingli Yang, Calvin Lau, Julia Voigtlaender-Bolz and Steffen-Sebastian Bolz
Lab Chip, 2010, 10, 2341-2349, DOI: 10.1039/C004675B
Simultaneous fabrication of PDMS through-holes for three-dimensional microfluidic applications
Bobak Mosadegh, Mayank Agarwal, Yu-suke Torisawa and Shuichi Takayama
Lab Chip, 2010, 10, 1983-1986, DOI: 10.1039/C003590D
Sickling of red blood cells through rapid oxygen exchange in microfluidic drops
Paul Abbyad, Pierre-Louis Tharaux, Jean-Louis Martin, Charles N. Baroud and Antigoni Alexandrou
Lab Chip, 2010, Advance Article, DOI: 10.1039/C004390G
Patterning microfluidic device wettability using flow confinement
Adam R. Abate, Julian Thiele, Marie Weinhart and David A. Weitz
Lab Chip, 2010, 10, 1774-1776, DOI: 10.1039/C004124F
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.