 Britta Weinhausen and Sarah Köster study hydrated eukaryotic cells device using X-rays in a new microfluidic device in their Technical Innovation, free to access for 4 weeks*.
Britta Weinhausen and Sarah Köster study hydrated eukaryotic cells device using X-rays in a new microfluidic device in their Technical Innovation, free to access for 4 weeks*.
The team at Georg-August-Universität Göttinghen, Germany, overcome some of the limitations of previous work to create a new type of device suitable for studying hydrated cells, with the possibility of applying it to living cells.
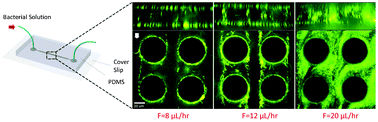
They use UV-curable glue (NOA 81) allowing sophisticated channel structures to be defined, two thin Kapton foils, with advantageous properties for X-ray studies, and a Si3N4 membrane window, upon which cells can be grown and fixed before the device is closed with a second membrane. Despite the small difference in electron density between the cellular material and water, they successfully produce X-ray dark-field images:
Microfluidic devices for X-ray studies on hydrated cells
Britta Weinhausen and Sarah Köster
DOI: 10.1039/C2LC41014A
 As mentioned in the LOC issue 2 blog, Luke Lee et al. at the University of California, Berkeley and Stanford University, featured on the front cover with their work on sorting stem cells using a cell cytometer based on the electrophysiological response to stimulus. As a HOT article that was featured on the cover, this article has been free to access* for 6 weeks from mid-December so get your skates on to read it in full in the next week:
As mentioned in the LOC issue 2 blog, Luke Lee et al. at the University of California, Berkeley and Stanford University, featured on the front cover with their work on sorting stem cells using a cell cytometer based on the electrophysiological response to stimulus. As a HOT article that was featured on the cover, this article has been free to access* for 6 weeks from mid-December so get your skates on to read it in full in the next week:
Label-free electrophysiological cytometry for stem cell-derived cardiomyocyte clusters
Frank B. Myers, Christopher K. Zarins, Oscar J. Abilez and Luke P. Lee
DOI: 10.1039/C2LC40905D
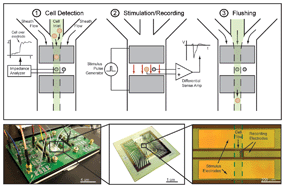
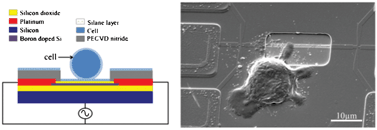 In this Technical Innovation, Rashid Bashir led a team of researchers from the USA, Sweden and Singapore in using silicon nanowires for rapid lysis of single cells by ultra-localized electroporation to release cell components for analysis. They integrate label-free magnetic manipulation to correctly position cells with the use of field effect transistors to apply an electric field for cell lysis.
In this Technical Innovation, Rashid Bashir led a team of researchers from the USA, Sweden and Singapore in using silicon nanowires for rapid lysis of single cells by ultra-localized electroporation to release cell components for analysis. They integrate label-free magnetic manipulation to correctly position cells with the use of field effect transistors to apply an electric field for cell lysis.
Their method eliminates the use of microfluidic trapping techniques, transparent substrates for optical tweezing and high voltages, amongst other requirements for previous devices.
This work is featured on the inside front cover of Issue 3, so it’s currently free to access for 6 weeks*. Do have a read of the article here:
Ultra-localized single cell electroporation using silicon nanowires
Nima Jokilaakso, Eric Salm, Aaron Chen, Larry Millet, Carlos Duarte Guevara, Brian Dorvel, Bobby Reddy, Amelie Eriksson Karlstrom, Yu Chen, Hongmiao Ji, Yu Chen, Ratnasingham Sooryakumar and Rashid Bashir
DOI: 10.1039/C2LC40837F
*Free access to individuals is provided through an RSC Publishing personal account. Registration is quick, free and simple