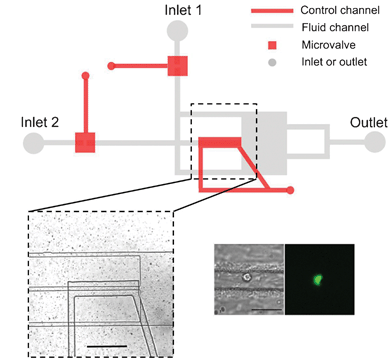
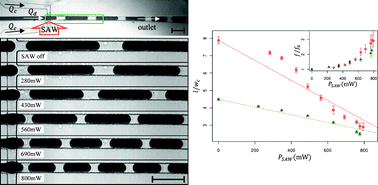
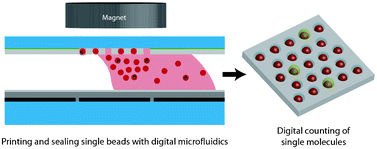
For the first time, refractive index sensitivity comparable to that of a commercial surface plasmon resonance sensor has been achieved in a nanohole array-based system, thanks to a team of Canadian engineers.
Surface plasmon resonance (SPR) sensors rely on the optical properties of thin gold films in order to measure changes in refractive index. SPR has been long been utilized for label-free biosensing, in which target molecules binding to a capture layer cause minute refractive index changes.1 Traditionally, these changes are measured via the reflectance spectrum of the gold film, requiring the light source and the detector to be placed at specific angles to the sample. This arrangement limits the types of experiments that can be carried out. However, if a gold film perforated with nanoholes is used, refractive index changes can be measured via the transmittance spectrum2, allowing the light source and detector to be arranged along a single light path, as in an ordinary spectrometer.
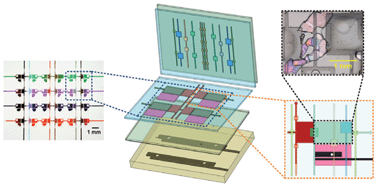
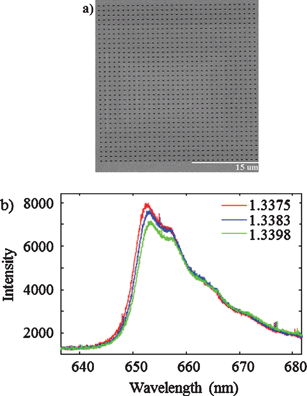 Prof. Reuven Gordon led a collaboration between the University of Victoria, the University of Ottawa, and Carleton University in an effort to improve the refractive index sensitivity of these nanohole array-based systems. The team made three key innovations: creating an ultra-smooth gold surface via template stripping, optimizing the shape of the nanoholes, and designing a support using a transparent material with a refraction index close to that of water. With these improvements, the researchers were able to match the sensitivity of commercial SPR systems using simple optical instrumentation. Because the nanohole substrates are used in a transmission geometry, they can be incorporated into optical and microfluidic systems in which SPR sensing has previously been unfeasible.
Prof. Reuven Gordon led a collaboration between the University of Victoria, the University of Ottawa, and Carleton University in an effort to improve the refractive index sensitivity of these nanohole array-based systems. The team made three key innovations: creating an ultra-smooth gold surface via template stripping, optimizing the shape of the nanoholes, and designing a support using a transparent material with a refraction index close to that of water. With these improvements, the researchers were able to match the sensitivity of commercial SPR systems using simple optical instrumentation. Because the nanohole substrates are used in a transmission geometry, they can be incorporated into optical and microfluidic systems in which SPR sensing has previously been unfeasible.
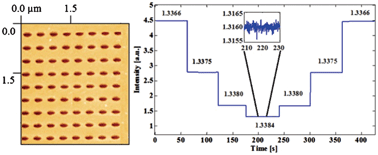
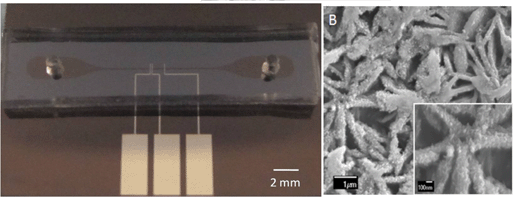
The image adapted from Figures 2b and 6a shows (a) the nanohole array and (b) its transmission spectra in various solutions.
References:
1. C. Boozer et al., Current Opinion in Biotechnology 2006, 17(4), 400–405, .
2. A. Krishnan et al., Optics Communications 2001, 200, 1–7, .
Read more in this HOT article from Lab on a Chip!
Atomically flat symmetric elliptical nanohole arrays in a gold film for ultrasensitive refractive index sensing
Gabriela Andrea Cervantes Tellez, Sa’ad Hassan, R. Niall Tait, Pierre Berini and Reuven Gordon
DOI: 10.1039/C3LC41411F
View the very best research in miniaturization from Canada in our themed issue Focus on Canada!
Katie Mayer is a post-doctoral researcher in the Walt Laboratory at Tufts University, USA