Fluorescence Activated Cell Sorting via a Focused Traveling Surface Acoustic Beam
Microfluidic Bead Trap as a Visual Bar for Quantitative Detection of Oligonucleotides
The design and engineering of biological systems — a field known as Synthetic Biology —has been heralded by many to be the defining technology of the 21st century. Just as electronics and computers have changed almost every facet of our day-to-day lives, the consequences of engineering microbes might also be as far reaching. Engineered bugs are already being developed to produce pharmaceuticals and fuels, monitor their environment, detect trace chemicals, degrade plastic waste, and perform computing operations.
Microbes, however, are considerably more complex — and hence less predictable — than electronic components. For this reason, one of the best strategies scientists have for engineering them is to use a brute force approach: try out as many different biodesigns as possible and see what works best.
Doing this with microbes is tricky. Designing and constructing a single engineered bacteria is labour intensive and can take weeks. To circumvent this, researchers have turned to cell-free systems: a collection of biomolecules that can reproduce biochemical features of interest, for example the synthesis of proteins from a genetic circuit. This greatly simplifies things as after all you are now merely handling what is essentially a sack of chemicals.
This is where it really gets interesting. As opposed to testing out different genetic circuits one-by-one in a test tube, Hori et al developed a microfluidic device which forms millions of droplets an hour – each droplet acts as a mini test tube, each containing a slightly different version of the circuit. The circuit was made up of three strands of DNA which interact with one another in a non-linear way to produce a Green Fluorescent Protein. The fact that it is non-linear means predicting how they interact is difficult and makes the tactic of trying out different combinations more appealing. Using microfluidics, the authors created a generic library by combining different ratios of these three components. By incubating the droplets and monitoring protein production levels, they were able to map out the parameter space to see which genetic circuit from their library worked best.
Although simply a proof-of-concept, this droplet microfluidic approach has shown to be incredibly powerful, and in addition to being used in genetic circuit design has the potential to rapidly explore large parameter spaces in other areas of chemistry and biology.
To download the full article for free* click the link below:
Cell-free extract based optimization of biomolecular circuits with droplet microfluidics
Yutaka Hori, Chaitanya Kantak, Richard M. Murray and Adam R. Abate
Lab Chip, 2017
DOI: 10.1039/C7LC00552K
*Free to access until 8th September 2017.
Yuval Elani is an EPSRC Research Fellow in the Department of Chemistry at Imperial College London. His research involves using microfluidics in bottom-up synthetic biology for biomembrane construction, artificial cell assembly, and for exploring bionterfaces between living and synthetic microsystems.
Euroanalysis 2017, will be held from 28th August – 1st September 2017, in Stockholm, Sweden.
Along the lines of the long traditions of Euroanalysis, the meeting will cover all aspects where analytical chemistry plays a role including fundamental and applied sciences. It will offer plenary and keynote presentations on cutting-edge topics by internationally renowned leaders of the field, followed by contributed talks and poster presentations to stimulate interdisciplinary discussions. In addition, a young researchers’ session will be organized to provide opportunities for and encourage Ph.D. students and postdocs to share their findings.
The conference has attracted about 500 participants from more than 30 countries, covering academia, governance and industry. The organisers hope that this will help to strengthen the networks between chemical societies and their members, working in diverse fields.
In addition to the lectures offered in the program, there are also opportunities to visit the exhibition, attend short courses and vendor’s seminars. A number of prominent analytical chemists will also be awarded.
A new 3D printer and resin formula enable 3D printing of channels on the microscale
There is no doubt that 3D printing is a hot technology with applications across all sectors of industry and life. The technology is attractive not only for its use in rapid prototyping, but also for its ability to form complex structures that could not easily be formed by other manufacturing techniques. There is also the added benefit of digital design—it is very easy to go from a CAD model to physical production without the need of additional tooling or equipment. For these reasons and others, 3D printing is an attractive manufacturing process for microfluidics.
Albert Folch has argued that 3D printing is the solution to the barriers preventing the translation of PDMS microfluidics to commercial applications. (Angew. Chem. Int. Ed. 2016, 55, 3862–3881.) However, printing resolution has limited the size of channels to the milli- and sub-millifluidic regimes. The challenge when it comes to microchannels is that commercial 3D printers are designed to form polymer features but microchannels, by their nature, are voids within bulk material. While many 3D printer manufacturers tout resolutions in the tens of micrometers, the reality is that the resolution of the void spaces can be several-fold larger. In their recent report, researchers at Brigham Young University have advanced 3D printing technology to print channels truly in the micro- regime.
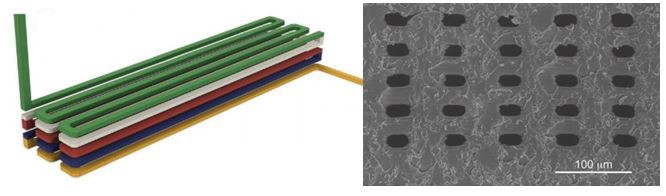
Left: Schematic illustration of 3D stacked serpentine channel design. Right: SEM image of 3D serpentine channel cross section.
Achieving true micro-scale printing resolution required innovation in both the 3D printer and the resin material. Gong et al. modified a digital light processing (DLP)-type 3D printer to include a high resolution light engine and a 385 nm UV LED light source. DLP printers project a pattern of light over the entire plane, crosslinking an entire layer in a single step. The light engine enables resolutions in the x-y plane of 7.6 µm and their choice of a 385 nm light source opened a wide range of possible UV-absorbers to them. Twenty different UV-absorbers were evaluated and 2-nitrophenyl phenyl sulfide (NPS) was chosen as having all the desired properties, with the main criterion of enabling smaller void sizes in the z-dimension. The final composition of the resin was a mixture of poly(ethylene glycol) diacrylate (PEGDA), NPS, and Irgacure 819. This mixture created structures that have low non-specific adsorption and had good resolution in the z-axis. Additionally, the structures could be photocured with a broad-spectrum UV lamp after printing.
Despite high-resolution printers and materials, a new printing regime also had to be developed to achieve the desired channel width. Because of scattering, the area of crosslinked resin tended to be smaller than the actual pixel size. This meant that channels, which are essentially voids of non-exposed space, came out wider than designed. The solution that Gong et al. devised was to perform two print patterns for each exposed layer. The first print pattern exposes the entire area, save for the channel void and the second exposure only projects a pattern along the channel walls. This method enabled reproducible printing of channels with a ~20 µm width.
So how soon can you expect to get your hands on a 3D printer capable of printing true microchannels? While this report has been very promising, it is currently the only printer and only resin formulation with these capabilities. According to Greg Nordin, the corresponding author, the main challenge is to figure out “how to get this into people’s hands in a convenient way so they don’t have to mess around much to get it to work.” Like any new technology, for it to be truly useful, users want to spend more time making and less time fiddling. Fortunately, there already are markets for printing small, intricate parts, such as jewelry and dentistry, so it’s only a matter of time before manufacturers catch on to the market for 3D printed microfluidics.
To download the full article for free* click the link below:
Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels
Hua Gong, Bryce P. Bickham, Adam T. Woolley and Gregory P. Nordin
Lab Chip, 2017
DOI: 10.1039/C7LC00644F
*Free to access until 29th August 2017.
About the Webwriter
Darius Rackus is a postdoctoral researcher at the University of Toronto working in the Wheeler Lab. His research interests are in combining sensors with digital microfluidics for healthcare applications.”
The first TISuMR Symposium on Tissue Culture and Magnetic Resonance will take place from 24th – 27th September, 2017, in Winchester, UK.
The culture of tissue under controlled conditions holds great promise for the future of medicine and the life sciences in general. While the study of cells provides detailed insight into biological processes at the molecular and supra-molecular scale, understanding many disease processes requires a more systemic approach.
Culture of tissues, either from organ samples or grown entirely in vitro, can provide insight at the organ system level, revealing not only the function of individual cell types, but also their interaction with each other as well as with the extracellular matrix.
Combining advanced microfluidic lab-on-a-chip approaches to tissue culture with non-invasive, in-situ NMR investigation of life processes is the central theme of the TISuMR project. This symposium will bring together clinical experts on liver pathology, researchers working on microfluidic tissue culture, and scientists developing novel magnetic resonance techniques, with the following aims:
The technical program will feature a number of leading scientists in the fields of hepatology, microfluidic technology, and magnetic resonance as keynote speakers. In addition, members of the TISuMR team will present their projects and early results.