Our skin perspires every day by about 2.5 million sweat glands under the dermis layer. Not all the sweat glands have the same function. While some glands release sweat through the opening of hair follicles−aka apocrine glands−, some others open directly onto the skin surface−aka eccrine glands−. The most prevalent type in the body is the eccrine glands, which produce interesting signaling subtleties for monitoring health. Actually, the sweat produced at different times contains the time-stamps of noteworthy information such as electrolytes, metabolites, micronutrients, hormones exogenous agents, each of which can change in concentration in the content of sweat with diet, stress level, hydration status, and physiologic or metabolic state. Monitoring these cues using appropriate sensors makes possible to track an individual’s health in real-time. Sweat analysis potentially complements or even obviates the need for approaches relying on puncturing the skin with needles.
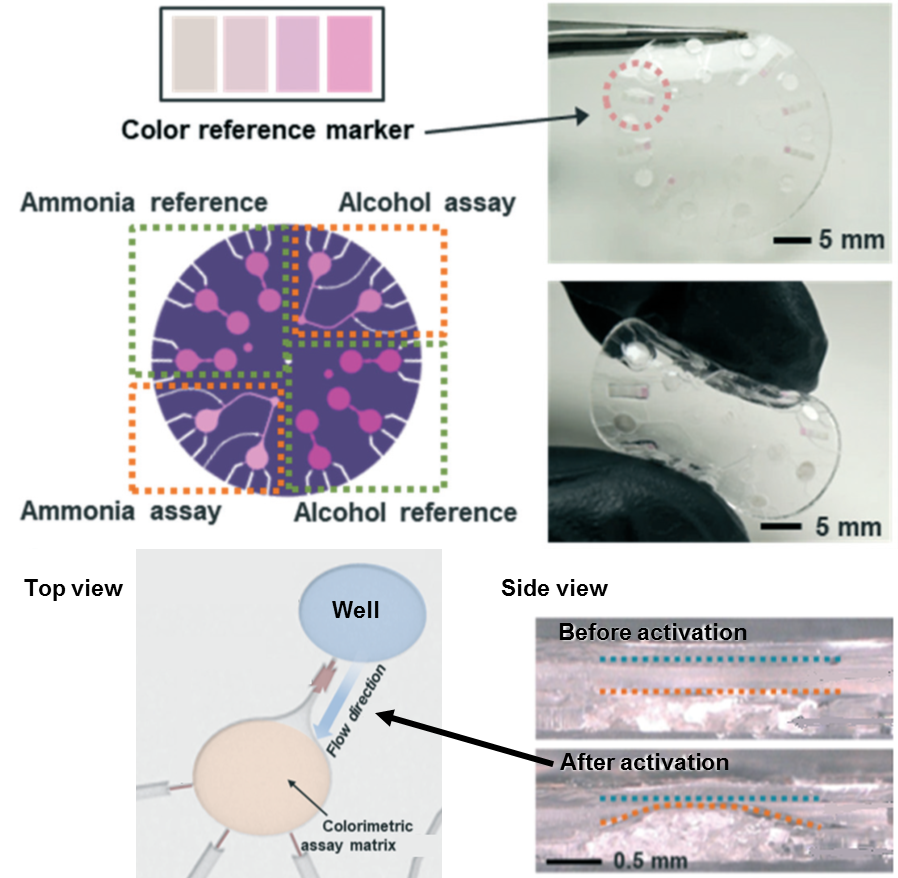
We encounter two major types of skin-interfaced measurement units in the literature: (1) equipped with electronics to power measurement units or run electrochemical measurements (2) colorimetric detection relying on no electronic sensors. Each type has different application areas. The colorimetric detection is particularly interesting as it relies on uniquely designed rapid chemical reactions between the sweat and the unit, in many cases with improved repeatability and accuracy. As a recent example of this kind, a research team from Northwestern University and the University of Illinois at Urbana-Champaign have introduced a skin sensor to detect signaling subtleties emanating from the skin. The promise of this work lies in the ability to control the reaction kinetics and the mixing of different reagents and samples in a user-operated device. This achievement was made possible by introducing a multi-layered microfluidic device platform containing stop valves and a super absorbent polymer to initiate colorimetric reactions for microliter volumes of ammonia and ethanol in microliter volumes of sweat.
The patch absorbs sweat via super absorbent polymer layers located subjacent to individual wells (Figure 1). The super absorbent polymer layer expands upon soaking sweat, activating a microfluidic mechanical pump that releases pre-loaded reaction buffers into the wells. The colorimetric reaction subsequently takes place (Figure 1). This pad was equipped for running ammonia and ethanol (alcohol) assays. But why two metabolites only? The patch is dedicated to monitoring alcohol testing in daily living. As mentioned in the paper, ammonia levels could serve as an index for hepatic encephalopathy diagnosis in subjects who are experiencing alcohol abuse. Hepatic encephalopathy refers to liver failure mostly caused by alcohol uptake. The researchers also tested the patch on volunteers resting in a warm bath after consuming alcoholic beverages to highlight the operational advantages of such patches. The authors note that this work has direct implications for sweat biomarker research, health monitoring in daily life, and simultaneous drug/alcohol testing.
To download the full article for free* click the link below:
Sung Bong Kim, Jahyun Koo, Jangryeol Yoon, Aurélie Hourlier-Fargette, Boram Lee,f Shulin Chen, Seongbin Jo, Jungil Choi, Yong Suk Oh, Geumbee Lee, Sang Min Won, Alexander J. Aranyosi, Stephen P. Lee, Jeffrey B. Model, Paul V. Braun, Roozbeh Ghaffari, Chulwhan Park and John A. Rogers, Lab Chip, 2020, Lab on a Chip Hot Articles
DOI: 10.1039/c9lc01045a
Burcu Gumuscu is a researcher in Mesoscale Chemical Systems Group at the University of Twente in the Netherlands. Her research interests include the development of microfluidic devices for quantitative analysis of proteins from single-cells, next-generation sequencing, compartmentalized organ-on-chip studies, and desalination of water on the microscale.