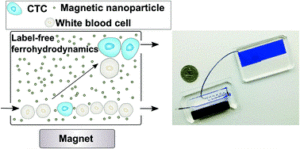
Cancers typically originate in one organ yet can spread to distant regions of the body forming secondary tumours called metastases. This happens as cells from the primary tumour migrate into the circulatory system and then travel to other organs. These cells, which are a very rare population within the circulatory system, are termed circulating tumor cells (CTCs). Because of their role in cancer biology, they have garnered a lot of interest lately. Their detection and isolation present several analytical challenges. For one, they are the proverbial “needle in a haystack”, with counts on the order of one CTC for every billion blood cells. This has traditionally led to a paradox: these rare cells are best handled in microscale systems but the world-to-chip mismatch limits microfluidic devices from rapidly processing the large (> 5 mL) samples necessary. Second, recent studies have revealed CTCs to be very heterogeneous populations, limiting the use of surface markers for labelling and capturing a broad range of CTCs. Because there is much still to learn about CTCs, there’s also an interest in recovering viable CTCs for further analysis. In their recent report, Zhao et al. demonstrate a microfluidic device capable of enriching CTCs using magnetic separation. But it’s not that typical magnetophoretic separations you may be familiar with!
Rather than using magnetic particles to bind to surface antigens and eventually separate out CTCs, they capitalize on a phenomenon known as “negative magnetophoresis”. Cells are suspended within a uniformly magnetic medium and application of a non-uniform magnetic field results in a magnetic buoyant force. (This is akin to how negative dielectrophoresis exerts a force on particles in a non-uniform electric field.) The advantage of this method is that the “working principle applies to every non-magnetic material,” according to Prof. Leidong Mao. “Naturally,” he thought “it could apply to CTC enrichment.” However, despite previous work separating different cell populations with negative magnetophoresis, moving to CTC enrichment is not so straightforward. CTC enrichment is the most challenging separation. “All previous applications in our group are with cells at high concentrations,” mentioned Prof. Mao. The main challenge in developing the chip was trying to preserve the characteristics of an “ideal” CTC enrichment device; one that could process a significant amount of blood quickly, have a high recovery rate of CTCs, give reasonable purity of isolated CTCs, and retain cell integrity and viability for further analysis.
With this method, heterogeneous populations of CTCs can be enriched as selection is size dependent rather than based on expression of certain surface markers. This also avoids the costs associated with traditional magnetic labelling – typically used to label and deplete the millions of white blood cells. The device is capable of working at flow rates of 5-7 mL/hr, which is what is necessary to process an entire blood sample and can achieve high recovery rates (>90%). While the authors report purities that appear low (10-12%), they are working on improving purity. One strategy they suggest in their report is to follow the route of the iChip and combine size based separations with magnetic WBC depletion.
To read the full paper for free*, click the link below:
Label-free ferrohydrodynamic cell separation of circulating tumor cells
DOI: 10.1039/C7LC00680B (Paper) Lab Chip, 2017, 17, 3097-3111
—————-
Darius Rackus is a postdoctoral researcher at the University of Toronto working in the Wheeler Lab. His research interests are in combining sensors with digital microfluidics for healthcare applications.
*free to access until 14th December 2017