Where life begins, the matter is structured at the micron scale by slotting every single molecule into its needful space. The molecules looped a bilipid membrane and nested more functional molecules inside, controlling the flows of energy in that smallest living cell. Every loop is a thicket of surprises on how much life can accomplish. High-precision measurements are a way to go to explore these small loops with immense information.
A cell requires the bilipid membrane to keep its functional molecules inside and separate itself from the surrounding environment. Bilipid membrane is a thin and flexible film made of water repellent and attractant molecules that form double layers in watery solutions where the water attractant heads line up outside and water repellent tails stay inside. Conditions on the two sides of a bilipid membrane (so, inside and outside of a cell) are often different because the presence and concentrations of surrounding ions and molecules will be different. Although the law of thermodynamics tries to equilibrate the conditions, ion pumps and proteins embedded in bilipid membranes pump molecules and ions in and out, in order to get the cell what it needs. We need a precise understanding of the nature of bilipid membranes to understand life, survival, and producing e.g., pharmaceuticals for survival or better living. Currently, multiple methodologies allow looking at a single cell or single chemical process step level. However, not that many allow analyzing the same phenomena with the same precision from different perspectives.
Voltage-dependent ion channels are a prominent example of the interest in an efficient methodology for multiple measurements. In essence, they allow for recording the electrical signals that surge as a response to activation of voltage-dependent channels, combined with simultaneous optical observation of structural changes and membrane dynamics. It is not a novel idea in the research world; however, reaching this goal has been challenging. Multiple methodologies have been proposed and tested, but frequently at a cost of a loss of precision or a rise in system complexity, making it hard to handle and more prone to error. The work published by Tobias Ensslen and Jan C. Behrends proposes a gracious solution that with a simple in-concept modification can achieve high optical resolution fluorescence microscopy while using a modified routinely used multielectrode-cavity array (MECA) (Figure 1).
While taking the original MECA design, a platform used for electrophysiological recordings for artificially formed lipid bilayers, a few alterations were implemented in order to accommodate the fluorescence imaging. The original device was designed with several novelties: (1) Silver/silver chloride electrodes and a gold conductive layer were used for detecting electrical signals emitted by an activated membrane. (2) The substrate for the fabricated system is coverslip glass instead of a microscope slide glass, which decreases the photons’ optical path from 500 µm to <170 µm. (3) The bottom electrodes were changed from a circular shape to a ring one, leaving a central opening for the light path. (4) The MECA-opto platform is also equipped with four apertures for membrane formation, which allow quickly switching the recording if a membrane is damaged or photobleaching in the recording site occurs. Altogether, these modifications enabled the acquisition of individually addressable electrophysiological and optical data without any additional equipment.
As a proof of concept study, the group demonstrated the molecular process of opening the voltage-dependant ion channel on the given membrane when activated by an antibacterial peptide, ceratotoxin-A. MECA-opto recordings allowed to determine the lifetimes of fluorophores with the results consistent with the available data regarding the stability of fluorophores. This is a promising work that can advance many studies at the molecular level and our understanding of the mechanisms of opening and closing ion channels.
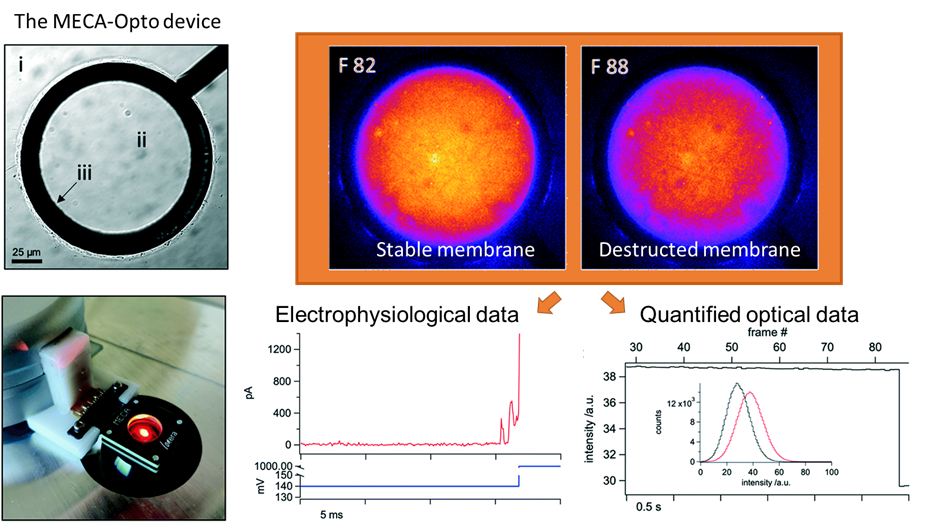
Figure 1. The MECA-Opto device can combine both electrophysiological and optical recordings to monitor free-standing membranes and membrane proteins.
To download the full article for free* click the link below:
Tobias Ensslen and Jan C. Behrends
Lab Chip, 2022, DOI: 10.1039/d2lc00357k
About the Web writers
 Oksana Savchak is a Ph.D. student in Biosensors and Devices Lab at the Eindhoven University of Technology in the Netherlands. She focuses on the development of microfluidic screening platforms to investigate cell-material interactions.
Oksana Savchak is a Ph.D. student in Biosensors and Devices Lab at the Eindhoven University of Technology in the Netherlands. She focuses on the development of microfluidic screening platforms to investigate cell-material interactions.
 Burcu Gumuscu is an assistant professor at Eindhoven University of Technology in the Netherlands, and the chair of the Biosensors and Devices Lab. She strives for the development, fabrication, and application of smart biomaterials to realize high-precision processing in high-throughput microfluidic settings. She specifically focuses on the design and development of lab-on-a-chip devices containing hydrogels for diversified life sciences applications.
Burcu Gumuscu is an assistant professor at Eindhoven University of Technology in the Netherlands, and the chair of the Biosensors and Devices Lab. She strives for the development, fabrication, and application of smart biomaterials to realize high-precision processing in high-throughput microfluidic settings. She specifically focuses on the design and development of lab-on-a-chip devices containing hydrogels for diversified life sciences applications.










