Even a tiny group of cells has the ability to populate a tumor in tissues. Determining cellular diversity and identifying these small cell groups gains importance when it comes to selection of treatment strategies. Tissue samples taken from patients are required to be dissociated into single-cell suspensions, therefore identification can be efficiently done at single-cell level using a powerful suite of technologies including flow cytometry, mass cytometry, and single cell RNA sequencing. However, breaking a tissue down to a single-cell suspension is not an easy task. The old-school way is to cut the tissue sample into small pieces with a blade and mechanically dissociated by vigorous shaking after the application of proteolytic enzymes. Large aggregates are removed by filtering the suspension through a strainer. This technique significantly increases the sample loss, drops the speed of the process and is not ideal for immediate downstream analysis.
In this month’s Lab on a Chip HOT article series, a group of researchers led by Dr Jered Haun at University of California Irvine presented a novel and simple approach that improves the quality of single-cell suspensions obtained from tissue samples using microfluidics. Jeremy Lombardo, a co-author of this article, explains that “the goal of this work was to fully replace manual intensive tissue dissociation protocols by using microfluidic devices.” The developed tool is a microdevice consisting of two nylon membranes, one with 25-50 µm mesh, and the other with 10-15 µm mesh, attached to micron-sized pores and microchannels. The device is made of laser-micromachined hard plastic (PET, aka. polyethylene terephthalate), which enables operation at high flow rates (>10 mL/min) when compared to PDMS (a silicon-based organic material). Also, the chip has multiple layers for connecting nylon mesh membranes at different levels (Figure 1).
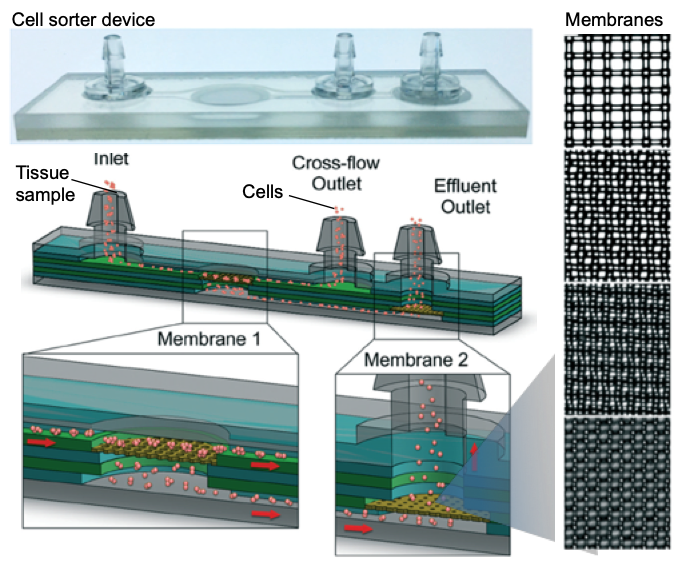
Figure 1. Microfluidic cell sorter device for tissue samples. The sketch shows the inner layers, consisting of two membranes for operating the device in direct or tangential filtration modes. Membrane mesh size can be adjusted to the cell size. Micrographs on the right show lattice network with several pore sizes used in this work. Pore sizes are (top to bottom) 50, 25, 15, 10 µm diameter.
Working principle of the cell sorter device
The inlet of the device connects to a microporous membrane to introduce tissue samples. The Sample passing through the membrane exits through the effluent outlet. It is also possible to direct a portion of the sample along the surface of the membrane that is connected to the cross-flow outlet. The device is either operated in a direct filtration regime to maximize sample recovery and processing speed, or in a tangential filtration regime to sweep larger tissue fragments and cell aggregates away to prevent clogging.
While the researchers initially hypothesized that under pressure-driven flow, cell and tissue aggregates might disaggregate as they pass through the membranes of the device, they were pleasantly surprised by the drastic level of single cell increases seen in the initial testing of these devices, says Jeremy Lombardo and adds “The hardest part in developing and testing this device was to find a combination of membrane pore sizes that could best dissociate cell aggregates and tissue without compromising cell viability. Thorough testing of various pore sizes and combinations were ultimately carried out with both cell line and murine tissue models before we settled on the final 50 and 15 μm pore sizes.”
Advantages, challenges and the future
The authors summarized the advantages of this platform for Lab on a Chip blog readers: “The device is extremely simple to operate as well as inexpensive to fabricate. It can easily be incorporated into many tissue dissociation applications for improved single cell yields as a standalone device but could also be easily integrated with other downstream microfluidic operations (cell sorting, detection etc.).” According to the authors, “in the current format of cell sorter device, cells that are very large in size would likely be difficult to process, as they would likely span multiple pores of the filters and be traditionally filtered away instead of dissociated.” Although seeming like a challenge, this can easily be addressed by adjusting the filter membrane pore sizes to accommodate these larger cell types.
For the future of the device, the authors indicate, “We are also currently working on integrating this device with upstream, larger scale tissue dissociation devices that we have developed previously to create a fully automatable microfluidic tissue dissociation platform.”
To download the full article for free* click the link below:
Microfluidic filter device with nylon mesh membranes efficiently dissociates cell aggregates and digested tissue into single cells
Xiaolong Qiu, Jeremy A. Lombardo, Trisha M. Westerhof, Marissa Pennell, Anita Ng, Hamad Alshetaiwi, Brian M. Luna, Edward L. Nelson, Kai Kessenbrock, Elliot E. Hui, and Jered B. Haun
Lab Chip, 2018, Lab on a Chip Recent Hot Articles
DOI: 10.1039/c8lc00507a
About the Webwriter
Burcu Gumuscu is a postdoctoral fellow in Herr Lab at UC Berkeley in the United States. Her research interests include development of microfluidic devices for quantitative analysis of proteins from single-cells, next generation sequencing, compartmentalized organ-on-chip studies, and desalination of water on the microscale.










